Osteoprotegerin ELISA
-
Category number
BI-20403
-
Method
Sandwich ELISA, HRP/TMB, 12×8-well detachable strips
-
Sample type
Serum, EDTA plasma, citrate plasma, heparin plasma
-
Sample volume
20 µl / well
-
Assay time
4 h / 1 h / 30 min
-
Sensitivity
0.07 pmol/l
-
Standard range
0 – 20 pmol/l (= 0 – 400 pg/ml)
-
Conversion factor
1 pg/ml = 0.05 pmol/l (MW: 19.9 kDa)
-
Specificity
Recombinant and endogenous human OPG.
-
Precision
In-between-run (n=12): ≤ 5 % CV
Within-run (n=5): ≤ 3 % CV
-
Cross-reactivity
The assay does not cross react with rat or mouse samples.
-
Use
CE marked – for IVD use in the EU
-
Validation Data
See validation data tab for: precision, accuracy, dilution linearity, values for healthy donors, etc
Product Overview
The Osteoprotegerin (OPG) immunoassay is a 4.5 hour, 96-well sandwich ELISA for the quantitative determination of OPG in human serum and plasma. The assay employs human serum-based standards to ensure the measurement of biologically reliable data.
Principle of the Assay
This kit is a sandwich enzyme immunoassay for the determination of OPG in human serum and plasma samples.
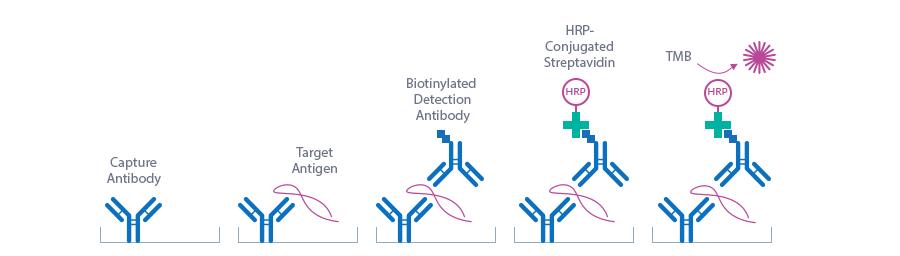
The figure below explains the principle of the Osteoprotegerin sandwich ELISA:
In a first step, assay buffer, standard/control/sample and detection antibody (biotinylated monoclonal mouse anti-human OPG) are pipetted into the wells of the microtiter strips, which are pre-coated with polyclonal goat anti-OPG antibody. OPG present in the standard/control/sample binds to the pre-coated antibody in the well and forms a sandwich with the detection antibody. In the washing step all non-specific unbound material is removed. In a second step, the conjugate (streptavidin-HRP) is pipetted into the wells and reacts with the detection antibody. After another washing step, the substrate (TMB, tetramethylbenzidine) is pipetted into the wells. The enzyme-catalyzed color change of the substrate is directly proportional to the amount of OPG. This color change is detectable with a standard microtiter plate reader. A dose response curve of the absorbance (optical density, OD at 450 nm) vs. standard concentration is generated. The concentration of OPG in the sample is determined directly from the dose response curve.
Typical Standard Curve
The figure below shows a typical standard curve for the OPG ELISA. The immunoassay is calibrated against recombinant human OPG:
ELISA Kit Components
|
Contents |
Description |
Quantity |
|
PLATE |
Polyclonal goat anti-OPG antibody pre-coated microtiter strips in strip holder, packed in aluminum bag with desiccant |
12 x 8 tests |
|
WASHBUF |
Wash buffer concentrate 20x, natural cap |
1 x 50 ml |
|
STD |
Standards 1-6, (0; 1.25; 2.5; 5; 10; 20 pmol/l), recombinant human OPG in human serum, white caps, ready to use |
6 x 300 µl |
|
CTRL |
Control, yellow cap, ready to use, exact concentration see label |
1 x 300 µl |
|
ASYBUF |
Assay buffer, red cap, ready to use |
1 x 25 ml |
|
AB |
Monoclonal mouse anti-OPG antibody, biotin labeled, green cap, yellow dye, ready to use |
1 x 7 ml |
|
CONJ |
Conjugate (streptavidin-HRP), amber bottle, amber cap, ready to use |
1 x 22 ml |
|
SUB |
Substrate (TMB solution), amber bottle, blue cap, ready to use |
1 x 22 ml |
|
STOP |
Stop solution, white cap, ready to use |
1 x 7 ml |
Storage instructions: All reagents of the OPG ELISA kit are stable at 4°C (2-8°C) until the expiry date stated on the label of each reagent.
Sample Collection & Storage
Serum, EDTA plasma, heparin plasma and citrate plasma are suitable for use in this assay. Do not change sample type during studies. We recommend duplicate measurements for all samples, standards and controls. The sample collection and storage conditions listed are intended as general guidelines.
Serum & Plasma
Collect venous blood samples in standardized serum separator tubes (SST) or standardized blood collection tubes using EDTA, heparin or citrate as an anticoagulant. For serum samples, allow samples to clot for 30 minutes at room temperature. Perform separation by centrifugation according to the tube manufacturer’s instructions for use. If this is not possible store the samples at 4°C (2-8°C) prior to centrifugation (up to one day). Assay the acquired samples immediately or aliquot and store at -25°C or lower. Lipemic or haemolyzed samples may give erroneous results. Samples can undergo at least four freeze-thaw cycles.
Reagent Preparation
Wash Buffer
|
1. |
Bring the WASHBUF concentrate to room temperature. Crystals in the buffer concentrate will dissolve at room temperature. |
|
2. |
Dilute the WASHBUF concentrate 1:20, e.g. 50 ml WASHBUF + 950 ml distilled or deionized water. Only use diluted WASHBUF when performing the assay. |
The diluted WASHBUF is stable up to one month at 4°C (2-8°C).
Sample Preparation
Bring samples to room temperature and mix samples gently to ensure the samples are homogenous. We recommend duplicate measurements for all samples.
Samples for which the OD value exceeds the highest point of the standard range can be diluted with STD1 or OPG negative human serum.
Assay Protocol
Read the entire protocol before beginning the assay.
|
1. |
Bring samples and reagents to room temperature (18-24°C). |
|
2. |
Mark positions for STD/CTRL/SAMPLE (standard/control/sample) on the protocol sheet. |
|
3. |
Take microtiter strips out of the aluminum bag. Store unused strips with desiccant at 4°C in the aluminum bag. Strips are stable until expiry date stated on the label. |
|
4. |
Pipette 150 µl ASYBUF (assay buffer, red cap) into each well. |
|
5. |
Add 20 µl STD/CTRL/SAMPLE (standard/control/sample) in duplicates into respective wells, swirl gently. |
|
6. |
Add 50 µl AB (biotinylated anti-OPG antibody, green cap) into each well, swirl gently. |
|
7. |
Cover the plate tightly and incubate for 4 hours at room temperature (18-24°C). |
|
8. |
Aspirate and wash wells 5x with 300 µl diluted WASHBUF (wash buffer). After the final wash, remove remaining WASHBUF by strongly tapping the plate against a paper towel. |
|
9. |
Add 200 µl CONJ (conjugate, amber cap) into each well. |
|
10. |
Cover tightly and incubate for 1 hour at room temperature (18-24°C). |
|
11. |
Aspirate and wash wells 5x with 300 µl diluted WASHBUF (wash buffer). After the final wash, remove remaining WASHBUF by strongly tapping the plate against a paper towel. |
|
12. |
Add 200 µl SUB (substrate, blue cap) into each well. |
|
13. |
Incubate for 30 min at room temperature (18-24°C) in the dark. |
|
14. |
Add 50 µl STOP (stop solution, white cap) into each well, swirl gently. |
|
15. |
Measure absorbance immediately at 450 nm with reference 630 nm, if available. |
Calculation of Results
Read the optical density (OD) of all wells on a plate reader using 450 nm wavelength (reference wavelength 630 nm). Construct a standard curve from the absorbance read-outs of the standards using commercially available software capable of generating a four-parameter logistic (4-PL) fit. Alternatively, plot the standards’ concentration on the x-axis against the mean absorbance for each standard on the y-axis and draw a best fit curve through the points on the graph. Curve fitting algorithms other than 4-PL have not been validated and will need to be evaluated by the user.
Obtain sample concentrations from the standard curve. If required, pmol/l can be converted into pg/ml by applying a conversion factor (1 pg/ml = 0.05 pmol/l; OPG MW: 19.9 kDa). Respective dilution factors have to be considered when calculating the final concentration of the sample.
The quality control (QC) protocol supplied with the kit shows the results of the final release QC for each kit at production date. Data for OD obtained by customers may differ due to various influences including the normal decrease of signal intensity throughout shelf life. However, this does not affect validity of results as long as an OD of 1.50 or more is obtained for STD6 and the value of the CTRL is within the target range (see label).
INFORMATION ON THE ANALYTE
OPG Protein
Osteoprotegerin (OPG) or osteoclast inhibitory factor (OCIF) is a glycoprotein of the tumor necrosis factor receptor superfamily encoded by the TNFRSF11B gene. OPG is synthesized as a monomer of 380 amino acids, assembled as a homodimer within the cell and then secreted mainly as a disulfide-linked homodimer into the extracellular compartment. OPG is produced by many different tissues and cell types including osteoblasts, breast tissue, vascular endothelial cells as well as B cells and dendritic cells in the immune system.
|
Molecular Weight |
19.9 kDa |
|
Cellular localization |
Extracellular |
|
Post-translational modifications |
Glycosylation |
|
Sequence similarities |
Member of the tumor necrosis factor receptor superfamily |
|
Alternative Names |
TNFRSF11B, OCIF, PDB5, TR1, tumor necrosis factor receptor superfamily member 11b, TNF receptor superfamily member 11b, osteoclastogenesis inhibitory factor |
|
Entrez/NCBI ID |
4982 link: https://www.ncbi.nlm.nih.gov/gene/4982 |
|
Genecards |
GC08M118923 link: https://www.genecards.org/cgi-bin/carddisp.pl?gene=TNFRSF11B |
|
OMIM |
602643 link: https://www.omim.org/entry/602643 |
|
PDB |
3URF link: http://www.rcsb.org/structure/3URF |
|
Protein Atlas |
TNFRSF11B link: https://www.proteinatlas.org/ENSG00000164761-TNFRSF11B/tissue |
|
Uniport ID |
O00300 link:https://www.uniprot.org/uniprot/O00300 |
OPG Function
OPG is a negative regulator of bone resorption by acting as decoy receptor for Receptor Activator of NF-κB Ligand (RANKL), thus neutralizing its function in osteoclastogenesis. It is also a decoy receptor for TRAIL and thereby inhibits apoptosis of mature osteoclasts. Hence, OPG is centrally involved in the regulation of bone resorbtion and formation. Since estrogen induces the expression of OPG in osteoblasts, post-menopausal decrease in estrogen levels can lead to the development of osteoporosis due to decreased OPG expression and enhanced RANKL-induced osteoclastogenesis. In contrast to estrogen, glucocorticoids induce the expression of RANKL and decrease the expressioin of OPG, which may lead to glucocorticoid-induced osteoporosis. Proinflammatory cytokines also increase the RANKL/OPG ratio and hence, induce bone loss in diseases like rheumatoid arthritis or periodontitis.
OPG has further been identified to promote tumor growth and survival by inducing tumor vascularization and inhibiting TRAIL-mediated tumor cell death. Dysregulation of the RANKL/OPG system is also common in metastatic bone disease, which is often observed in disseminated cancers of breast, prostate and lung. In multiple myeloma, reduced OPG levels and increased osteoclastogenesis cause the formation of osteolytic bone lesions.
Increased concentrations of OPG, especially in the context of diabetes and chronic kidney disease, are associated with vascular calcification and increased cardiovascular risk.
-
Bone Diseases
-
Osteoporosis
-
Rheumatoid arthritis
-
Osteoarthritis
-
Juvenile idiopathic arthritis
-
Juvenile Paget's disease
-
Paget's disease of the bone
-
Glucocorticoid-induced osteoporosis
-
Ankylosing spondylitis
-
Periodontitis
-
-
Cardiovascular Diseases
-
Vascular calcification
-
Peripheral artery disease
-
Heart failure
-
Coronary artery disease
-
-
Oncology
-
Multiple myeloma
-
Hairy cell leukemia
-
Breast cancer
-
Prostate Cancer
-
Lung Cancer
-
Bone metastasis
-
Osteosarcoma
-
Tumor-induced osteomalacia
-
Literature
-
Kenkre, J.S., Bassett, J., 2018. Ann. Clin. Biochem. 55, 308–327.
PMID:29368538
-
The Osteoprotegerin: multiple partners for multiple functions.
Baud’huin, M., Duplomb, L., Teletchea, S., Lamoureux, F., Ruiz-Velasco, C., Maillasson, M., Redini, F., Heymann, M.-F., Heymann, D., 2013. Cytokine Growth Factor Rev. 24, 401–409.
PMID:23827649
-
Bone Remodelling Markers in Rheumatoid Arthritis.
Fardellone, P., Séjourné, A., Paccou, J., Goëb, V., 2014. Mediators Inflamm 2014.
PMID:24839355
-
Glucocorticoid-induced osteoporosis.
Briot, K., Roux, C., 2015. RMD Open 1, e000014.
PMCID: PMC4613168
PMID:26509049
-
Inflammatory bone loss: pathogenesis and therapeutic intervention.
Redlich, K., Smolen, J.S., 2012. Nature Reviews Drug Discovery 11, 234–250.
PMID:22378270
-
The role of biomarkers in the management of bone-homing malignancies.
D’Oronzo, S., Brown, J., Coleman, R., 2017. J Bone Oncol 9, 1–9.
PMCID: PMC5602513
PMID:28948139
-
Rachner, T.D., Kasimir-Bauer, S., Göbel, A., Erdmann, K., Hoffmann, O., Browne, A.J., Wimberger, P., Rauner, M., Hofbauer, L.C., Kimmig, R., Bittner, A.-K., 2018. Clin. Cancer Res.
PMID:30425091
-
Aberrant regulation of RANKL/OPG in women at high risk of developing breast cancer.
Kiechl, S., Schramek, D., Widschwendter, M., Fourkala, E.-O., Zaikin, A., Jones, A., Jaeger, B., Rack, B., Janni, W., Scholz, C., Willeit, J., Weger, S., Mayr, A., Teschendorff, A., Rosenthal, A., Fraser, L., Philpott, S., Dubeau, L., Keshtgar, M., Roylance, R., Jacobs, I.J., Menon, U., Schett, G., Penninger, J.M., 2016. Oncotarget 8, 3811–3825.
PMCID: PMC5354797
PMID:28002811
-
Infante, M., Fabi, A., Cognetti, F., Gorini, S., Caprio, M., Fabbri, A., 2019. J. Exp. Clin. Cancer Res. 38, 12.
PMCID: PMC6325760
PMID:30621730
-
Serum osteoprotegerin and sex steroid levels in patients with prostate cancer.
Varsavsky, M., Reyes-Garcia, R., Avilés Perez, M.D., Gonzalez Ramírez, A.R., Mijan, J.L., Muñoz-Torres, M., 2012. J. Androl. 33, 594–600.
PMID:21903971
-
Role of the RANK/RANKL Pathway in Multiple Myeloma
Raje, N.S., Bhatta, S., Terpos, E., 2019. Clin. Cancer Res. 25, 12–20.
PMID:30093448
-
Rochette, L., Meloux, A., Rigal, E., Zeller, M., Cottin, Y., Vergely, C., 2018. Pharmacol. Ther. 182, 115–132.
PMID:28867452
All Biomedica ELISAs are validated according to international FDA/ICH/EMEA guidelines. For more information about our validation guidelines, please refer to our quality page and published validation guidelines and literature.
-
ICH Q2 (R1) Validation of Analytical Procedures: Text and Methodology
-
EMEA/CHMP/EWP/192217/2009 Guideline on Validation of Bioanalytical Methods
- Bioanalytical Method Validation, Guidance for Industry, FDA, May 2018
Calibration
The OPG immunoassay is calibrated against recombinant human OPG protein (AA 22-194 of Uniprot ID# O00300).
Detection Limit & Sensitivity
To determine the sensitivity of the OPG ELISA, experiments measuring the lower limit of detection (LOD) and the lower limit of quantification (LLOQ) were conducted.
The LOD, also called the detection limit, is the lowest point at which a signal can be distinguished above the background signal, i.e. the signal that is measured in the absence of OPG, with a confidence level of 99%. It is defined as the mean back calculated concentration of standard 1 (0 pmol/l of OPG, five independent measurements) plus three times the standard deviation of the measurements.
The LLOQ, or sensitivity of an assay, is the lowest concentration at which an analyte can be accurately quantified. The criteria for accurate quantification at the LLOQ are an analyte recovery between 75 and 125% and a coefficient of variation (CV) of less than 25%. To determine the LLOQ, standard 2, i.e. the lowest standards containing OPG, is diluted, measured two times and its concentration is back calculated. The lowest dilution, which meets both criteria, is reported as the LLOQ.
The following values were determined for the OPG ELISA:
|
LOD |
0.07 pmol/l |
|
LLOQ |
0.08 pmol/l |
Precision
The precision of an ELISA is defined as its ability to measure the same concentration consistently within the same experiments carried out by one operator (within-run precision or repeatability) and across several experiments using the same samples but conducted by several operators at different locations using different ELISA lots (in-between-run precision or reproducibility).
Within-Run Precision (Repeatability or Intra-Assay Precision)
Within-run precision was assessed by measuring two samples of known concentrations five times within one OPG ELISA kit lot by one operater.
|
ID |
n |
Mean OPG [pmol/l] |
SD [pmol/l] |
CV [%] |
|
S1 |
5 |
3.2 |
0.05 |
2 |
|
S2 |
5 |
10.1 |
0.34 |
3 |
In-Between Run Precision (Inter-Assay Precision)
In-between-run precision was assessed by measuring two samples twelfe times within two OPG ELISA kit lots by three different operaters.
|
ID |
n |
Mean OPG [pmol/l] |
SD [pmol/l] |
CV [%] |
|
S1 |
12 |
3.2 |
0.10 |
3 |
|
S2 |
12 |
9.9 |
0.5 |
5 |
Accuracy
The accuracy of an ELISA is defined as the precision with which it can recover samples of known concentrations.
The recovery of the OPG ELISA was measured by adding recombinant OPG to human samples containing a known concentration endogenous OPG. The %recovery of the spiked concentration was calculated as the percentage of measured compared over the expected value. All our ELISAs are expected to have %recovery rates within 15% of the nominal value of the sample.
This table shows the summary of the recovery experiments in the OPG ELISA in different sample matrices:
|
% Recovery |
|||||||
|
Sample Matrix |
n |
+ 2 pmol/l |
+5 pmol/l |
+ 10 pmol/l |
|||
|
Mean |
Range |
Mean |
Range |
Mean |
Range |
||
|
Serum |
3 |
95 |
85-106 |
105 |
94-111 |
95 |
89-99 |
|
EDTA plasma |
3 |
100 |
99-102 |
93 |
83-113 |
108 |
93-119 |
|
Citrate plasma |
3 |
88 |
82-96 |
89 |
76-100 |
109 |
99-120 |
|
Heparin plasma |
3 |
91 |
67-118 |
82 |
70-95 |
94 |
84-110 |
Data showing recovery (R [%]) of recombinant OPG in human serum samples:
|
OPG [pmol/l] |
% Recovery |
||||||||||||
|
Sample Matrix |
ID |
Reference |
+2 pmol/l |
+5 pmol/l |
+10 pmol/l |
+2 pmol/l |
+5 pmol/l |
+10 pmol/l |
|||||
|
Serum |
s1 |
3.8 |
5.5 |
8.1 |
13.3 |
106 |
94 |
99 |
|||||
|
Serum |
s2 |
2.6 |
4.5 |
8.2 |
12.3 |
95 |
110 |
97 |
|||||
|
Serum |
s3 |
3.5 |
5.2 |
9.0 |
12.4 |
85 |
111 |
89 |
|||||
|
|
|
|
|
|
|
95 |
105 |
95 |
Mean | ||||
|
|
|
|
|
|
|
85 |
94 |
89 |
Min | ||||
|
|
|
|
|
|
|
106 |
111 |
99 |
Max | ||||
Data showing recovery of recombinant OPG in human EDTA plasma samples:
|
OPG [pmol/l] |
% Recovery |
||||||||||||
|
Sample Matrix |
ID |
Reference |
+2 pmol/l |
+5 pmol/l |
+10 pmol/l |
+2 pmol/l |
+5 pmol/l |
+10 pmol/l |
|||||
|
EDTA plasma |
s1 |
3.3 |
5.2 |
7.4 |
14.4 |
99% |
83% |
112% |
|||||
|
EDTA plasma |
s2 |
2.5 |
4.6 |
6.7 |
14.4 |
102% |
83% |
119% |
|||||
|
EDTA plasma |
s3 |
3.3 |
5.3 |
8.9 |
12.5 |
100% |
113% |
93% |
|||||
|
|
|
|
|
|
|
100% |
93% |
108% |
Mean | ||||
|
|
|
|
|
|
|
99 |
83 |
93 |
Min | ||||
|
|
|
|
|
|
|
102 |
113 |
119 |
Max | ||||
Data showing recovery of recombinant OPG in a human citrate plasma sample:
|
OPG [pmol/l] |
% Recovery |
||||||||||||
|
Sample Matrix |
ID |
Reference |
+2 pmol/l |
+5 pmol/l |
+10 pmol/l |
+2 pmol/l |
+5 pmol/l |
+10 pmol/l |
|||||
|
Citrate plasma |
s1 |
2.7 |
4.4 |
7.7 |
12.6 |
85% |
100% |
99% |
|||||
|
Citrate plasma |
s2 |
2.1 |
4.0 |
5.9 |
12.9 |
96% |
76% |
108% |
|||||
|
Citrate plasma |
s3 |
2.8 |
4.4 |
7.3 |
14.8 |
82% |
90% |
120% |
|||||
|
|
|
|
|
|
|
88% |
89% |
109% |
Mean | ||||
|
|
|
|
|
|
|
82 |
76 |
99 |
Min | ||||
|
|
|
|
|
|
|
96 |
100 |
120 |
Max | ||||
|
OPG [pmol/l] |
% Recovery |
||||||||||||
|
Sample Matrix |
ID |
Reference |
+2 pmol/l |
+5 pmol/l |
+10 pmol/l |
+2 pmol/l |
+5 pmol/l |
+10 pmol/l |
|||||
|
Citrate plasma |
s1 |
3.1 |
4.4 |
6.6 |
11.5 |
67% |
70% |
84% |
|||||
|
Citrate plasma |
s2 |
2.3 |
4.6 |
6.4 |
13.3 |
118% |
82% |
110% |
|||||
|
Citrate plasma |
s3 |
3.1 |
4.9 |
7.9 |
12.0 |
87% |
95% |
89% |
|||||
|
|
|
|
|
|
|
91% |
82% |
94% |
Mean | ||||
|
|
|
|
|
|
|
67 |
70 |
84 |
Min | ||||
|
|
|
|
|
|
|
118 |
95 |
110 |
Max | ||||
Dilution Linearity & Paralellism
Tests of dilution linearity and parallelism ensure that both endogenous and recombinant samples containing OPG behave in a dose dependent manner and are not affected by matrix effects. Dilution linearity assesses the accuracy of measurements in diluted clinical samples spiked with known concentrations of recombinant analyte. By contrast, parallelism refers to dilution linearity in clinical samples and provides evidence that the endogenous analyte behaves in same way as the recombinant one. Dilution linearity and parallelism are assessed for each sample type and are considered acceptable if the results are within ±20% of the expected concentration.
Dilution linearity was assessed by serially diluting human serum samples spiked with 6 pmol/l recombinant OPG with standard 1.
The table below shows the mean recovery and range of serially diluted recombinant OPG in serum samples:
|
% Recovery of recombinant OPG in diluted samples |
|||
|
Sample Matrix |
n |
1+1 |
|
|
Mean |
Range |
||
|
Serum |
8 |
96 |
84-116 |
Data showing dilution linearity of 6 pmol/l recombinant OPG spiked into human serum samples (reference) containing endogenous OPG:
|
OPG [pmol/l] |
% Recovery |
|||||||
|
Sample Matrix |
ID |
Reference |
+6 pmol/l |
1+1 |
1+1 |
|||
|
Serum |
s1 |
3.7 |
10.7 |
5.2 |
98% |
|||
|
Serum |
s2 |
2.3 |
6.7 |
3.9 |
116% |
|||
|
Serum |
s3 |
4.4 |
12.8 |
5.1 |
80% |
|||
|
Serum |
s4 |
3.4 |
12.0 |
5.1 |
86% |
|||
|
Serum |
s5 |
3.3 |
9.4 |
5.0 |
107% |
|||
|
Serum |
s6 |
3.6 |
10.3 |
5.2 |
101% |
|||
|
Serum |
s7 |
4.4 |
11.6 |
4.9 |
84% |
|||
|
Serum |
s8 |
4.7 |
10.9 |
5.2 |
96% |
|||
|
|
|
|
|
|
96 |
Mean | ||
|
|
|
|
|
|
80 |
Min | ||
|
|
|
|
|
|
116 |
Max | ||
Parallelism was assessed by serially diluting human serum samples containing endogenous OPG with standard 1.
The table below shows the mean recovery and range of serially diluted endogenous OPG in human serum samples:
|
% Recovery of endogenous OPG in diluted samples |
|||||||
|
Sample Matrix |
n |
1+1 |
1+3 |
1+7 |
|||
|
Mean |
Range |
Mean |
Range |
Mean |
Range |
||
|
Serum |
3 |
98 |
92-102 |
90 |
86-94 |
87 |
79-86 |
Data showing dilution linearity of endogenous OPG in human serum samples:
|
OPG [pmol/l] |
% Recovery |
||||||||||||
|
Sample Matrix |
ID |
Reference |
1+1 |
1+3 |
1+7 |
1 + 1 |
1 + 3 |
1 + 7 |
|||||
|
Serum |
s1 |
8.2 |
4.1 |
1.9 |
0.9 |
99% |
94% |
86% |
|||||
|
Serum |
s2 |
3.6 |
1.9 |
0.8 |
0.4 |
102% |
92% |
95% |
|||||
|
Serum |
s3 |
6.7 |
3.1 |
1.4 |
0.7 |
92% |
86% |
79% |
|||||
|
|
|
|
|
|
|
98 |
90 |
87 |
Mean | ||||
|
|
|
|
|
|
|
92 |
86 |
79 |
Min | ||||
|
|
|
|
|
|
|
102 |
94 |
95 |
Max | ||||
Specificity
The OPG ELISA recognizes human endogenous and recombinant OPG. It detects monomeric and dimeric OPG as well as OPG-RANKL complexes. The assay does not cross-react with rat or mouse samples.
Sample Stability
The stability of endogenous OPG was tested by comparing OPG measurements in samples that had undergone four freeze-thaw (F/T) cycles.
For freeze-thaw experiments, samples were collected according to the supplier’s instruction using blood collection devices and stored at -80°C. Reference samples were freeze-thawed once. The mean recovery of sample concentration after four freeze-thaw cycles is 101%.
|
OPG [pmol/l] |
% Recovery after 4 (F/T) cycles |
|||||||
|
Sample Matrix |
ID |
Reference |
2x |
3x |
4x |
|||
|
Serum |
s1 |
2.8 |
2.8 |
3.7 |
2.5 |
86% |
||
|
Serum |
s2 |
3.1 |
3.8 |
4.1 |
3.7 |
119 |
||
|
Serum |
s3 |
5.0 |
5.4 |
5.3 |
5.1 |
103 |
||
|
Serum |
s4 |
2.6 |
3.0 |
2.6 |
2.5 |
94 |
||
|
|
|
|
|
|
|
101 |
Mean | |
Samples can undergo at least up to four freeze-thaw cycles.
Sample Values
OPG Values in Apparently Healthy Individuals
To provide expected values for circulating OPG, a panel of samples from apparently healthy donors was tested.
A summary of the results is shown below:
|
OPG [pmol/l] |
||
|
Sample Matrix |
n |
Mean |
|
Serum |
60 |
2.7 |
|
EDTA plasma |
6 |
2.2 |
|
Citrate plasma |
5 |
2.3 |
|
Heparin plasma |
7 |
2.3 |
It is recommended to establish the normal range for each laboratory.
Matrix Comparison
To assess whether all tested matrices behave the same way in the OPG ELISA, concentrations of OPG were measured in serum, EDTA, citrate and heparin plasma samples prepared from four apparently healthy donors. Each individual donated blood in all tested sample matrices.
A summary table of OPG levels in various sample matrices is shown below:
|
|
OPG [pmol/l] |
|
||||
|
Sample ID |
Serum |
EDTA plasma |
Citrate plasma |
Heparin plasma |
% CV |
|
|
#1 |
2.8 |
3.4 |
2.4 |
2.9 |
14 |
|
|
#2 |
2.8 |
2.8 |
2.6 |
2.3 |
9 |
|
|
#3 |
2.1 |
3.1 |
2.3 |
2.6 |
17 |
|
|
#4 |
0.9 |
1.0 |
0.8 |
0.8 |
11 |
|
|
13 |
Mean |
|||||
- Imbalance of osteoprotegerin/receptor activator of nuclear factor-κB ligand and oxidative stress in patients with obstructive sleep apnea-hypopnea syndrome.
Ma, X.-R., Wang, Y., Sun, Y.-C., 2019. Chinese Medical Journal 132, 25.
- The role of sclerostin/dickkopf-1 and receptor activator of nuclear factor kB ligand/osteoprotegerin signalling pathways in the development of osteoporosis in patients with haemophilia A and B: A cross-sectional study.
Anagnostis, P., Vakalopoulou, S., Christoulas, D., Paschou, S.A., Papatheodorou, A., Garipidou, V., Kokkoris, P., Terpos, E., 2018. Haemophilia 24, 316–322.
- LRP5 gene polymorphisms and radiographic joint damage in rheumatoid arthritis patients.
Bernardes, M., Durães, C., Oliveira, A., Martins, M.J., Lucas, R., Costa, L., Pereira, J.G., Ramos, I., Machado, J.C., Simões-Ventura, F., 2018. Osteoporosis International.
- GH prevents adipogenic differentiation of mesenchymal stromal stem cells derived from human trabecular bone via canonical Wnt signaling.
Bolamperti, S., Signo, M., Spinello, A., Moro, G., Fraschini, G., Guidobono, F., Rubinacci, A., Villa, I., 2018. Bone 112, 136–144.
PMID:29694926
- Study of bone metabolism and angiogenesis in patients undergoing high-dose chemotherapy/autologous hematopoietic stem cell transplantation.
Boutsikas, G., Terpos, E., Papatheodorou, A., Tsirkinidis, P., Tsirigotis, P., Meletiou, A., Lalou, E., Telonis, V., Zannou, A., Kanellopoulos, A., Galani, Z., Stefanou, A., Tsaftaridis, P., Viniou, N.-A., Panayiotidis, P., Kyrtsonis, M.-C., Meletis, J., Vassilakopoulos, T.P., Angelopoulou, M.K., 2018. European Journal of Haematology 100, 131–139.
- Monocytes from male patients with ankylosing spondylitis display decreased osteoclastogenesis and decreased RANKL/OPG ratio.
Caparbo, V.F., Saad, C.G.S., Moraes, J.C., de Brum-Fernandes, A.J., Pereira, R.M.R., 2018. Osteoporos Int.
PMID:30006885
- Circulating Soluble Receptor Activator of Nuclear Factor Kappa B Ligand and C-C Motif Ligand 3 Correlate With Survival in Patients With Waldenström Macroglobulinemia.
Eleutherakis-Papaiakovou, E., Kastritis, E., Gavriatopoulou, M., Christoulas, D., Roussou, M., Ntanasis-Stathopoulos, I., Kanellias, N., Papatheodorou, A., Dimopoulos, M.A., Terpos, E., 2018. Clin Lymphoma Myeloma Leuk 18, 431–437.
PMID:29685422
- Poor Vitamin K Status Is Associated With Low Bone Mineral Density and Increased Fracture Risk in End-Stage Renal Disease.
Evenepoel, P., Claes, K., Meijers, B., Laurent, M., Bammens, B., Naesens, M., Sprangers, B., Pottel, H., Cavalier, E., Kuypers, D., 2018. J. Bone Miner. Res.
PMID:30427544
- RANKL/RANK/OPG Axis Is Deregulated in the Cerebrospinal Fluid of Multiple Sclerosis Patients at Clinical Onset.
Glasnović, A., Stojić, M., Dežmalj, L., Tudorić-Đeno, I., Romić, D., Jeleč, V., Vrca, A., Vuletić, V., Grčević, D., 2018. Neuroimmunomodulation 25, 23–33.
PMID:29920500
- Clinical predictive biomarkers for normoalbuminuric diabetic kidney disease.
Gohda, T., Nishizaki, Y., Murakoshi, M., Nojiri, S., Yanagisawa, N., Shibata, T., Yamashita, M., Tanaka, K., Yamashita, Y., Suzuki, Y., Kamei, N., 2018. Diabetes Res. Clin. Pract. 141, 62–68.
PMID:29729375
- Cardiovascular autonomic neuropathy and bone metabolism in Type 1 diabetes.
Hansen, C.S., Theilade, S., Lajer, M., Hansen, T.W., Rossing, P., 2018. Diabet. Med.
PMID:29999549
- Increased frequency of temporal acoustic window failure in rheumatoid arthritis: a manifestation of altered bone metabolism?
Kardos, Z., Oláh, C., Sepsi, M., Sas, A., Kostyál, L., Bóta, T., Bhattoa, H.P., Hodosi, K., Kerekes, G., Tamási, L., Bereczki, D., Szekanecz, Z., 2018. Clin. Rheumatol. 37, 1183–1188.
PMID:29383454
- Comparison of bone turnover markers in peripheral blood and bone marrow aspirate.
Ornstrup, M.J., Kjær, T.N., Harsløf, T., Jørgensen, N.R., Pedersen, S.B., Langdahl, B.L., 2018. Bone 116, 315–320.
PMID:30176391
- Associations of osteoprotegerin with coronary artery calcification among women with systemic lupus erythematosus and healthy controls.
Poornima, I.G., Shields, K., Kuller, L.H., Manzi, S.M., Ramsey-Goldman, R., Richardson, C., Rhew, E., Dunlop, D.D., Song, J., Edmundowicz, D., Kondos, G.T., Carr, J.J., Langman, C.B., Price, H., Chung, A.H., Santelices, L.B., Mackey, R.H., 2018. Lupus 961203317751060. PMCID: PMC6026582
PMID:29310535
- Prognostic value of RANKL/OPG serum levels and disseminated tumor cells in non-metastatic breast cancer.
Rachner, T.D., Kasimir-Bauer, S., Göbel, A., Erdmann, K., Hoffmann, O., Browne, A.J., Wimberger, P., Rauner, M., Hofbauer, L.C., Kimmig, R., Bittner, A.-K., 2018. Clin. Cancer Res.
PMID:30425091
- Rheumatoid Arthritis Magnetic Resonance Imaging Score Predicts Therapy Response: Results of the German ArthroMark Cohort.
Sewerin, P., Le, L., Vordenbäumen, S., Schleich, C., Sengewein, R., Brinks, R., Pongratz, G., Bleck, E., Lesch, J., Mansmann, U., Schneider, M., Ostendorf, B., 2018. The Journal of Rheumatology 45, 753–759.
- Consolidation therapy with the combination of bortezomib and lenalidomide (VR) without dexamethasone in multiple myeloma patients after transplant: Effects on survival and bone outcomes in the absence of bisphosphonates.
Terpos, E., Kastritis, E., Ntanasis-Stathopoulos, I., Christoulas, D., Papatheodorou, A., Eleutherakis-Papaiakovou, E., Kanellias, N., Fotiou, D., Ziogas, D.C., Migkou, M., Roussou, M., Trougkakos, I.P., Gavriatopoulou, M., Dimopoulos, M.A., 2018. Am. J. Hematol.
PMID:30592079
- Effects of Biliopancreatic Diversion on Bone Turnover Markers and Association with Hormonal Factors in Patients with Severe Obesity.
Turcotte, A.-F., Grenier-Larouche, T., Ung, R.-V., Simonyan, D., Carreau, A.-M., Carpentier, A.C., Mac-Way, F., Michou, L., Tchernof, A., Biertho, L., Lebel, S., Marceau, S., Gagnon, C., 2018. Obes Surg.
PMID:30478790
- Cardiovascular disease and bone loss—new research in identifying common disease pathophysiologies and predictors.
West, S.L., O’Donnell, E., 2018. AME Medical Journal 3
- Gene variants of osteoprotegerin, estrogen-, calcitonin- and vitamin D-receptor genes and serum markers of bone metabolism in patients with Gaucher disease type 1.
Zimmermann, A., Popp, R.A., Rossmann, H., Bucerzan, S., Nascu, I., Leucuta, D., Weber, M.M., Grigorescu-Sido, P., 2018. Therapeutics and Clinical Risk Management Volume 14, 2069–2080.
- Serum serotonin levels and bone in rheumatoid arthritis patients.
Bernardes, M., Vieira, T., Lucas, R., Pereira, J., Costa, L., Simões-Ventura, F., Martins, M.J., 2017a. Rheumatology International 37, 1891–1898.
- Myocardial Perfusion in Rheumatoid Arthritis Patients: Associations with Traditional Risk Factors and Novel Biomarkers.
Bernardes, M., Vieira, T.S., Martins, M.J., Lucas, R., Costa, L., Pereira, J.G., Ventura, F., Martins, E., 2017b. BioMed Research International 2017, 1–9.
- Calcification Propensity of Serum is Independent of Excretory Renal Function.
Bielesz, B., Reiter, T., Marculescu, R., Gleiss, A., Bojic, M., Kieweg, H., Cejka, D., 2017. Sci Rep 7, 17941.PMCID: PMC5738386
PMID:29263429
- Markers of bone metabolism during 14 days of bed rest in young and older men.
Buehlmeier, J., Frings-Meuthen, P., Mohorko, N., Lau, P., Mazzucco, S., Ferretti, J.L., Biolo, G., Pisot, R., Simunic, B., Rittweger, J., 2017 10
- Clearance of Sclerostin, Osteocalcin, Fibroblast Growth Factor 23, and Osteoprotegerin by Dialysis.
Carlson, N., Mortensen, O.H., Axelsen, M., Pedersen, R.S., Heaf, J.G., 2017. Blood Purif. 44, 122–128.
- Biochemical markers of bone metabolism during early orthodontic tooth movement with aligners.
Castroflorio, T., Gamerro, E.F., Caviglia, G.P., Deregibus, A., 2017. Angle Orthod 87, 74–81.
PMID:27409364
- Osteoprotegerin, RANKL, ADMA, and Fetuin-A serum levels in children with type I diabetes mellitus.
Chrysis, D., Efthymiadou, A., Mermigka, A., Kritikou, D., Spiliotis, B.E., 2017. Pediatr Diabetes 18, 277–282.
PMID:27028343
- Effect of recent spinal cord injury on the OPG/RANKL system and its relationship with bone loss and the response to denosumab therapy.
Gifre, L., Ruiz-Gaspà, S., Carrasco, J.L., Portell, E., Vidal, J., Muxi, A., Monegal, A., Guañabens, N., Peris, P., 2017. Osteoporos Int 28, 2707–2715.
PMID:28580511
- Autoantibodies to Osteoprotegerin are Associated with Low Hip Bone Mineral Density and History of Fractures in Axial Spondyloarthritis: A Cross-Sectional Observational Study.
Hauser, B., Zhao, S., Visconti, M.R., Riches, P.L., Fraser, W.D., Piec, I., Goodson, N.J., Ralston, S.H., 2017. Calcif. Tissue Int. 101, 375–383. PMCID: PMC5587630
PMID:28534161
- Receptor Activator of Nuclear Transcription Factor NF-κB (RANK), Its Ligand RANKL, and Natural Inhibitor of RANKL Osteoprotegerin (OPG) in the Blood Serum of Patients with Primary Bone Tumors.
Kushlinskii, N.E., Gershtein, E.S., Solov’ev, Y.N., Timofeev, Y.S., Babkina, I.V., Dolinkin, A.O., Zuev, A.A., Kostyleva, O.I., 2017. Bull. Exp. Biol. Med. 163, 478–481.
PMID:28853064
- Weekly oral bisphosphonates over 2 years prevent bone loss in cardiac transplant patients.
Lange, U., Classen, K., Müller-Ladner, U., Richter, M., 2017. Clin Transplant 31.
PMID:28940569
- Long-Term Effects of Severe Burn Injury on Bone Turnover and Microarchitecture.
Muschitz, G.K., Schwabegger, E., Fochtmann, A., Baierl, A., Kocijan, R., Haschka, J., Gruther, W., Schanda, J.E., Resch, H., Rath, T., Pietschmann, P., Muschitz, C., 2017. Journal of Bone and Mineral Research 32, 2381–2393.
- Effect of Tumor Necrosis Factor Inhibitor Therapy on Osteoclasts Precursors in Rheumatoid Arthritis.
Perpétuo, Inês P., Caetano-Lopes, J., Rodrigues, A.M., Campanilho-Marques, R., Ponte, C., Canhão, H., Ainola, M., Fonseca, J.E., 2017a. BioMed Research International 2017, 1–10.
- Methotrexate and low-dose prednisolone downregulate osteoclast function by decreasing receptor activator of nuclear factor-κβ expression in monocytes from patients with early rheumatoid arthritis.
Perpétuo, Inês Pedro, Caetano-Lopes, J., Rodrigues, A.M., Campanilho-Marques, R., Ponte, C., Canhão, H., Ainola, M., Fonseca, J.E., 2017. RMD Open 3, e000365. PMCID: PMC5604603
PMID:28955481
- Ankylosing Spondylitis Patients Have Impaired Osteoclast Gene Expression in Circulating Osteoclast Precursors.
Perpétuo, Inês P., Caetano-Lopes, J., Vieira-Sousa, E., Campanilho-Marques, R., Ponte, C., Canhão, H., Ainola, M., Fonseca, J.E., 2017b. Frontiers in Medicine 4.
- Serum irisin and myostatin levels after 2 weeks of high-altitude climbing.
Śliwicka, E., Cisoń, T., Kasprzak, Z., Nowak, A., Pilaczyńska-Szcześniak, Ł., 2017. PLoS ONE 12, e0181259. PMCID: PMC5521782
PMID:28732027
- Atypical skeletal manifestations of rickets in a familial hypocalciuric hypercalcemia patient.
Wu, B., Wang, O., Jiang, Y., Li, M., Xing, X., Xia, W., 2017. Bone Res 5, 17001. PMCID: PMC5486235
PMID:28690912
- Hajdu Cheney Syndrome; report of a novel NOTCH2 mutation and treatment with denosumab.
Adami, G., Rossini, M., Gatti, D., Orsolini, G., Idolazzi, L., Viapiana, O., Scarpa, A., Canalis, E., 2016. Bone 92, 150–156. PMCID: PMC5056853
PMID:27592446
- Daily Intake of Milk Enriched with n-3 Fatty Acids, Oleic Acid, and Calcium Improves Metabolic and Bone Biomarkers in Postmenopausal Women.
Fonolla-Joya, J., Reyes-García, R., García-Martín, A., López-Huertas, E., Muñoz-Torres, M., 2016. J Am Coll Nutr 35, 529–536.
PMID:27463412
- Effect of Osteoprotegerin and Dickkopf-Related Protein 1 on Radiological Progression in Tightly Controlled Rheumatoid Arthritis.
Gómez-Vaquero, C., Martín, I., Loza, E., Carmona, L., Ivorra, J., Narváez, J.A., Hernández-Gañán, J., Alía, P., Narváez, J., 2016. PLOS ONE 11, e0166691.
- Aberrant regulation of RANKL/OPG in women at high risk of developing breast cancer.
Kiechl, S., Schramek, D., Widschwendter, M., Fourkala, E.-O., Zaikin, A., Jones, A., Jaeger, B., Rack, B., Janni, W., Scholz, C., Willeit, J., Weger, S., Mayr, A., Teschendorff, A., Rosenthal, A., Fraser, L., Philpott, S., Dubeau, L., Keshtgar, M., Roylance, R., Jacobs, I.J., Menon, U., Schett, G., Penninger, J.M., 2016. Oncotarget 8, 3811–3825. PMCID: PMC5354797
PMID:28002811
- Subclinical arteriosclerosis and osteoprotegerin levels in a population with systemic lupus erythematous in the south of Europe.
López-Robles, C., Rios-Fernández, R., Callejas-Rubio, J.-L., Moreno-Escobar, E., Martín-DeLaFuente, P., Ortego-Centeno, N., 2016. Lupus 25, 781–782.
PMID:26768749
- Osteolytic lesions, cytogenetic features and bone marrow levels of cytokines and chemokines in multiple myeloma patients: Role of chemokine (C-C motif) ligand 20.
Palma, B.D., Guasco, D., Pedrazzoni, M., Bolzoni, M., Accardi, F., Costa, F., Sammarelli, G., Craviotto, L., De Filippo, M., Ruffini, L., Omedè, P., Ria, R., Aversa, F., Giuliani, N., 2016. Leukemia 30, 409–416.
- Associations between OPG and RANKL polymorphisms, vertebral fractures, and abdominal aortic calcification in community-dwelling older subjects: the Sao Paulo Ageing & Health Study (SPAH).
Pereira, R.M.R., Figueiredo, C.P., Cha, C.C., Caparbo, V.F., Oliveira, R.M., Franco, A.S., Menezes, P.R., de Castro, I., Onuchic, L.F., 2016. Osteoporos Int 27, 3319–3329.
PMID:27311721
- Differences in biochemical bone markers by diabetes type and the impact of glucose.
Starup-Linde, J., Lykkeboe, S., Gregersen, S., Hauge, E.-M., Langdahl, B.L., Handberg, A., Vestergaard, P., 2016. Bone 83, 149–155.
PMID:26555635
- Assessment of OPG, RANKL, bone turnover markers serum levels and BMD after treatment with strontium ranelate and ibandronate in patients with postmenopausal osteoporosis.
Stuss, M., Sewerynek, E., Król, I., Stępień-Kłos, W., Jędrzejczyk, S., 2016. Endokrynol Pol 67, 174–184.
PMID:26884284
- Role of Osteoprotegerin and Receptor Activator of Nuclear Factor-κB Ligand in Bone Loss Related to Advanced Chronic Obstructive Pulmonary Disease.
Ugay, L., Kochetkova, E., Nevzorova, V., Maistrovskaia, Y., 2016. Chin. Med. J. 129, 1696–1703. PMCID: PMC4960959
PMID:27411457
- Chronic skin inflammation leads to bone loss by IL-17-mediated inhibition of Wnt signaling in osteoblasts.
Uluçkan, Ö., Jimenez, M., Karbach, S., Jeschke, A., Graña, O., Keller, J., Busse, B., Croxford, A.L., Finzel, S., Koenders, M., van den Berg, W., Schinke, T., Amling, M., Waisman, A., Schett, G., Wagner, E.F., 2016. Sci Transl Med 8, 330ra37.
PMID:27089206
- Craniometaphyseal dysplasia with obvious biochemical abnormality and rickets-like features.
Wu, B., Jiang, Y., Wang, O., Li, M., Xing, X.-P., Xia, W.-B., 2016. Clin. Chim. Acta 456, 122–127.
PMID:26820766
- Osteoprotegerin as a marker of atherosclerosis in type 1 and type 2 diabetic patients.
Alkaç, C., Alkaç, B., Akbaş, F., Aral, H., Karagöz, Y., Altunoğlu, E.G., 2015. Turk J Med Sci 45, 1306–1311 PMID: 26775387
- Osteocalcin is a predictor for diabetes mellitus in postmenopausal women and correlated with oral intake of vitamin k.
Asadipooya, K., Graves, L., Lukert, B.P., Kalantarhormozi, M., Assadi, M., Ostovar, A., Larijani, B., Nabipour, I., 2015. Mediterranean Journal of Nutrition and Metabolism 8, 231–241.
- RANKL and OPG gene polymorphisms: associations with vertebral fractures and bone mineral density in premenopausal systemic lupus erythematosus.
Bonfá, A.C., Seguro, L.P.C., Caparbo, V., Bonfá, E., Pereira, R.M.R., 2015. Osteoporos Int 26, 1563–1571.
PMID:25609157
- The effect of tocilizumab on bone mineral density, serum levels of Dickkopf-1 and bone remodeling markers in patients with rheumatoid arthritis.
Briot, K., Rouanet, S., Schaeverbeke, T., Etchepare, F., Gaudin, P., Perdriger, A., Vray, M., Steinberg, G., Roux, C., 2015. Joint Bone Spine 82, 109–115.
- A Prospective Study on Inflammatory Cytokines and Bone Metabolism Mediators in Patients Affected by Rheumatoid and Psoriatic Arthritis treated with Adalimumab.
Chimenti, M.S., Maria Morello, P.C., 2015. Journal of Arthritis 04.
- Mechanisms of enhanced osteoclastogenesis in girls and young women with Turner’s Syndrome.
Faienza, M.F., Brunetti, G., Ventura, A., Piacente, L., Messina, M.F., De Luca, F., Ciccarelli, M., Oranger, A., Mori, G., Natale, M.P., Gigante, M., Ranieri, E., Gesualdo, L., Colucci, S., Cavallo, L., Grano, M., 2015. Bone 81, 228–236.
PMID:26208797
- Decreased bone cortical density at the forearm in subjects with subclinical peripheral arterial disease.
Gaudio, A., Muratore, F., Fiore, V., Rapisarda, R., Signorelli, S.S., Fiore, C.E., 2015. Osteoporos Int 26, 1747–1753.
PMID:25672808
- Enhanced IL-6 trans-signaling in pulmonary arterial hypertension and its potential role in disease-related systemic damage.
Jasiewicz, M., Knapp, M., Waszkiewicz, E., Ptaszynska-Kopczynska, K., Szpakowicz, A., Sobkowicz, B., Musial, W.J., Kaminski, K.A., 2015. Cytokine 76, 187–192.
PMID:26163998
- Osteoprotegerin is a significant prognostic factor for overall survival in patients with primary systemic amyloidosis independent of the Mayo staging.
Kastritis, E., Gavriatopoulou, M., Dimopoulos, M.A., Eleutherakis-Papaiakovou, E., Kanellias, N., Roussou, M., Pamboucas, C., Toumanidis, S.T., Terpos, E., 2015. Blood Cancer J 5, e319. PMCID: PMC4648482
PMID:26047389
- Cystatin C as a potential predictor of osteoprotegerin levels in healthy men, a cross-sectional, observational study.
Kulcsar-Jakab, E., Petho, Z., Pap, Z., Kalina, E., Foldesi, R., Balogh, A., Antal-Szalmas, P., Bhattoa, H.P., 2015. BMC Musculoskelet Disord 16, 227.
PMID:26311162
- Effects of Antitumor Necrosis Factor Therapy on Osteoprotegerin, Neopterin, and sRANKL Concentrations in Patients with Rheumatoid Arthritis.
Kurz, K., Herold, M., Russe, E., Klotz, W., Weiss, G., Fuchs, D., 2015. Dis. Markers 2015, 276969. PMCID: PMC4631883
PMID:26576067
- Role of plaque calcification regulators osteoprotegerin and matrix Gla-proteins in stable angina and acute myocardial infarction.
Margonato, A., Gorla, R., Macchi, A., Buzzetti, F., Franzoni, I., Pedrigi, M.C., Rosa, I., Sirtori, M., Villa, I., Rubinacci, A., 2015. J Cardiovasc Med (Hagerstown) 16, 156–162.
PMID:24566391
- Role of osteoprotegerin in vascular disorders of the end-stage renal disease patients.
Moldovan, D., Kacso, I.M., Rusu, C., Potra, A., Bondor, C.I., Moldovan, I., Patiu, I.M., Vladutiu, D., Caprioara, M.G., 2015. Biomarkers 20, 116–122.
PMID:25585925
- Switching from tenofovir to abacavir in HIV-1-infected patients with low bone mineral density: changes in bone turnover markers and circulating sclerostin levels.
Negredo, E., Diez-Pérez, A., Bonjoch, A., Domingo, P., Pérez-Álvarez, N., Gutierrez, M., Mateo, G., Puig, J., Echeverría, P., Escrig, R., Clotet, B., 2015. J. Antimicrob. Chemother. 70, 2104–2107.
PMID:25769303
- Serum osteoprotegerin is associated with pulse pressure in kidney transplant recipients.
Nemeth, Z.K., Mardare, N.G., Czira, M.E., Deak, G., Kiss, I., Mathe, Z., Remport, A., Ujszaszi, A., Covic, A., Molnar, M.Z., Mucsi, I., 2015. Sci Rep 5, 14518. PMCID: PMC4602220
PMID:26459001
- Association of osteoprotegerin and bone loss after adjuvant chemotherapy in early-stage breast cancer.
Oostra, D.R., Lustberg, M.B., Reinbolt, R.E., Pan, X., Wesolowski, R., Shapiro, C.L., 2015. Mol. Cell. Endocrinol. 402, 51–56. PMCID: PMC4316829
PMID:25575458
- Adipose tissue, estradiol levels, and bone health in obese men with metabolic syndrome.
Ornstrup, M.J., Kjær, T.N., Harsløf, T., Stødkilde-Jørgensen, H., Hougaard, D.M., Cohen, A., Pedersen, S.B., Langdahl, B.L., 2015. Eur. J. Endocrinol. 172, 205–216.
PMID:25416724
- Selected pro-inflammatory cytokines, bone metabolism, osteoprotegerin, and receptor activator of nuclear factor-kB ligand in girls with anorexia nervosa.
Ostrowska, Z., Ziora, K., Oświęcimska, J., Marek, B., Świętochowska, E., Kajdaniuk, D., Strzelczyk, J., Cieślicka, A., Wołkowska-Pokrywa, K., Kos-Kudła, B., 2015. Endokrynol Pol 66, 313–321.
PMID:26323468
- CD14 and TNFα single nucleotide polymorphisms are candidates for genetic biomarkers of peri-implantitis.
Rakic, M., Petkovic-Curcin, A., Struillou, X., Matic, S., Stamatovic, N., Vojvodic, D., 2015. Clin Oral Investig 19, 791–801.
PMID:25217276
- Osteoprotegerin concentrations in patients with suspected reversible myocardial ischemia: observations from the Akershus Cardiac Examination (ACE) 1 Study.
Røysland, R., Røsjø, H., Høiseth, A.D., Gullestad, L., Badr, P., Kravdal, G., Omland, T., 2015. Cytokine 73, 122–127.
PMID:25748834
- Serum fetuin-A levels and abdominal aortic calcification in healthy men — The STRAMBO study.
Schoppet, M., Rauner, M., Benner, J., Chapurlat, R., Hofbauer, L.C., Szulc, P., 2015. Bone 79, 196–202.
- Lower P1NP serum levels: a predictive marker of bone loss after 1 year follow-up in premenopausal systemic lupus erythematosus patients.
Seguro, L.P.C., Casella, C.B., Caparbo, V.F., Oliveira, R.M., Bonfa, A., Bonfa, E., Pereira, R.M.R., 2015. Osteoporosis International 26, 459–467.
- The association of bone turnover markers with pro- and anti-inflammatory adipokines in patients with gestational diabetes.
Telejko, B., Kalejta, K., Kuzmicki, M., Wawrusiewicz-Kurylonek, N., Lipinska, D., Pliszka, J., Wilk, J., Zielinska, A., Sobota, A., Szamatowicz, J., Kretowski, A., Gorska, M., 2015. Ann Agric Environ Med 22, 307–312.
PMID:26094529
- Serum sclerostin in high-activity adult patients with juvenile idiopathic arthritis.
Brabnikova-Maresova, K., Jarosova, K., Pavelka, K., Stepan, J.J., 2014. Arthritis Research & Therapy 16.
- Independent relationship of osteoprotegerin concentrations with endothelial activation and carotid atherosclerosis in patients with severe rheumatoid arthritis.
Dessein, P.H., López-Mejias, R., González-Juanatey, C., Genre, F., Miranda-Filloy, J.A., Llorca, J., González-Gay, M.A., 2014. J. Rheumatol. 41, 429–436.
PMID:24488413
- Osteoclastogenic potential of peripheral blood mononuclear cells in cleidocranial dysplasia.
Faienza, M.F., Ventura, A., Piacente, L., Ciccarelli, M., Gigante, M., Gesualdo, L., Colucci, S., Cavallo, L., Grano, M., Brunetti, G., 2014. Int J Med Sci 11, 356–364. PMCID: PMC3936030
PMID:24578613
- Early effects of tumor necrosis factor inhibition on bone homeostasis after soluble tumor necrosis factor receptor use.
Lim, M.J., Kwon, S.R., Joo, K., Son, M.J., Park, S.-G., Park, W., 2014. The Korean Journal of Internal Medicine 29, 807.
- Serum levels of sclerostin, Dickkopf-1, and secreted frizzled-related protein-4 are not changed in individuals with high bone mass causing mutations in LRP5.
Simpson, C.A., Foer, D., Lee, G.S., Bihuniak, J., Sun, B., Sullivan, R., Belsky, J., Insogna, K.L., 2014. Osteoporosis International 25, 2383–2388.
- Severe abdominal aortic calcification in older men is negatively associated with DKK1 serum levels: the STRAMBO study.
Szulc, P., Schoppet, M., Rachner, T.D., Chapurlat, R., Hofbauer, L.C., 2014. J. Clin. Endocrinol. Metab. 99, 617–624.
PMID:24276456
- The combination of lenalidomide and dexamethasone reduces bone resorption in responding patients with relapsed/refractory multiple myeloma but has no effect on bone formation: Final results on 205 patients of the Greek myeloma study group.
Terpos, Evangelos, Christoulas, D., Kastritis, E., Katodritou, E., Papatheodorou, A., Pouli, A., Kyrtsonis, M.-C., Michalis, E., Papanikolaou, X., Gkotzamanidou, M., Koulieris, E., Gavriatopoulou, M., Zervas, K., Dimopoulos, M.A., on behalf of the Greek Myeloma Study Group, 2014. American Journal of Hematology 89, 34–40.
- VTD consolidation, without bisphosphonates, reduces bone resorption and is associated with a very low incidence of skeletal-related events in myeloma patients post ASCT.
Terpos, E, Christoulas, D., Kastritis, E., Roussou, M., Migkou, M., Eleutherakis-Papaiakovou, E., Gavriatopoulou, M., Gkotzamanidou, M., Kanellias, N., Manios, E., Papadimitriou, C., Dimopoulos, M.A., 2014. Leukemia 28, 928–934.
- Comparative Effect of Zoledronic Acid Versus Denosumab on Serum Sclerostin and Dickkopf-1 Levels of Naive Postmenopausal Women With Low Bone Mass: A Randomized, Head-to-Head Clinical Trial.
Anastasilakis, A.D., Polyzos, S.A., Gkiomisi, A., Bisbinas, I., Gerou, S., Makras, P., 2013. The Journal of Clinical Endocrinology & Metabolism 98, 3206–3212.
- Traditional and novel bone remodeling markers in premenopausal and postmenopausal women.
Botella, S., Restituto, P., Monreal, I., Colina, I., Calleja, A., Varo, N., 2013. J. Clin. Endocrinol. Metab. 98, E1740-1748.
PMID:24001743
- High dickkopf-1 levels in sera and leukocytes from children with 21-hydroxylase deficiency on chronic glucocorticoid treatment.
Brunetti, G., Faienza, M.F., Piacente, L., Ventura, A., Oranger, A., Carbone, C., Benedetto, A.D., Colaianni, G., Gigante, M., Mori, G., Gesualdo, L., Colucci, S., Cavallo, L., Grano, M., 2013. American Journal of Physiology-Endocrinology and Metabolism 304, E546–E554.
- Low-dose prednisolone in early rheumatoid arthritis inhibits collagen type I degradation by matrix metalloproteinases as assessed by serum 1CTP--a possible mechanism for specific inhibition of radiological destruction.
Engvall, I.-L., Svensson, B., Boonen, A., van der Heijde, D., Lerner, U.H., Hafström, I., BARFOT study group, 2013. Rheumatology (Oxford) 52, 733–742.
PMID:23275387
- Activation of the receptor activator of the nuclear factor-κB ligand pathway during coronary bypass surgery: comparison between on- and off-pump coronary artery bypass surgery procedures.
Galeone, A., Brunetti, G., Rotunno, C., Oranger, A., Colucci, S., de Luca Tupputi Schinosa, L., Zallone, A., Grano, M., Paparella, D., 2013. Eur J Cardiothorac Surg 44, e141-147.
PMID:23671202
- OPG and sRANKL serum levels and incident hip fracture in postmenopausal Caucasian women in the Women’s Health Initiative Observational Study.
LaCroix, A.Z., Jackson, R.D., Aragaki, A., Kooperberg, C., Cauley, J.A., Chen, Z., Leboff, M.S., Duggan, D., Wactawski-Wende, J., 2013. Bone 56, 474–481. PMCID: PMC3832355
PMID:23735608
- An independent positive relationship between the serum total osteocalcin level and fat-free mass in healthy premenopausal women.
Liu, J., Zhao, H., Zhao, L., Chen, Y., Zhang, L., Tao, B., Sun, L., Zhao, Y., Wang, W., Xu, M., Chen, J., Ning, G., 2013. J. Clin. Endocrinol. Metab. 98, 2146–2152.
PMID:23553865
- Synovial membrane immunohistology in early-untreated rheumatoid arthritis reveals high expression of catabolic bone markers that is modulated by methotrexate.
Revu, S., Neregård, P., af Klint, E., Korotkova, M., Catrina, A.I., 2013. Arthritis Res. Ther. 15, R205. PMCID: PMC3978873
PMID:24295447
- Juvenile paget’s disease in an Iranian kindred with vitamin D deficiency and novel homozygous TNFRSF11B mutation.
Saki, F., Karamizadeh, Z., Nasirabadi, S., Mumm, S., McAlister, W.H., Whyte, M.P., 2013. J. Bone Miner. Res. 28, 1501–1508. PMCID: PMC3663917
PMID:23322328
- Bone metabolism compensates for the delayed growth in small for gestational age neonates.
Tenta, R., Bourgiezi, I., Aliferis, E., Papadopoulou, M., Gounaris, A., Skouroliakou, M., 2013. Organogenesis 9, 55–59. PMCID: PMC3674041
PMID:23538775
- Sclerostin and its association with physical activity, age, gender, body composition, and bone mineral content in healthy adults.
Amrein, K., Amrein, S., Drexler, C., Dimai, H.P., Dobnig, H., Pfeifer, K., Tomaschitz, A., Pieber, T.R., Fahrleitner-Pammer, A., 2012. J. Clin. Endocrinol. Metab. 97, 148–154.
PMID:21994959
- Physical training increases osteoprotegerin in postmenopausal women.
Bergström, I., Parini, P., Gustafsson, S.A., Andersson, G., Brinck, J., 2012. J. Bone Miner. Metab. 30, 202–207.
PMID:21823052
- Diets higher in dairy foods and dietary protein support bone health during diet- and exercise-induced weight loss in overweight and obese premenopausal women.
Josse, A.R., Atkinson, S.A., Tarnopolsky, M.A., Phillips, S.M., 2012. J. Clin. Endocrinol. Metab. 97, 251–260.
PMID:22049177
- Changes of serum soluble receptor activator for nuclear factor-κB ligand after glucocorticoid therapy reflect regulation of its expression by osteoblasts.
Kaneko, K., Kusunoki, N., Hasunuma, T., Kawai, S., 2012. J. Clin. Endocrinol. Metab. 97, E1909-1917. PMCID: PMC3462941
PMID:22791764
- Serum Osteoprotegerin, RANKL, and Dkk-1 Levels in Adults with Langerhans Cell Histiocytosis.
Makras, P., Polyzos, S.A., Anastasilakis, A.D., Terpos, E., Kanakis, G., Schini, M., Papatheodorou, A., Kaltsas, G.A., 2012. The Journal of Clinical Endocrinology & Metabolism 97, E618–E621.
- RANK-RANKL-OPG in hemophilic arthropathy: from clinical and imaging diagnosis to histopathology.
Melchiorre, D., Milia, A.F., Linari, S., Romano, E., Benelli, G., Manetti, M., Guiducci, S., Ceccarelli, C., Innocenti, M., Carulli, C., Civinini, R., Morfini, M., Matucci-Cerinic, M., Ibba-Manneschi, L., 2012. J. Rheumatol. 39, 1678–1686.
PMID:22753650
- Plasma pyridoxal-5-phosphate is inversely associated with systemic markers of inflammation in a population of U.S. adults.
Sakakeeny, L., Roubenoff, R., Obin, M., Fontes, J.D., Benjamin, E.J., Bujanover, Y., Jacques, P.F., Selhub, J., 2012. J. Nutr. 142, 1280–1285. PMCID: PMC3374666
PMID:22623384
- Serum Level of the Phosphaturic Factor FGF23 Is Associated with Abdominal Aortic Calcification in Men: The STRAMBO Study.
Schoppet, M., Hofbauer, L.C., Brinskelle-Schmal, N., Varennes, A., Goudable, J., Richard, M., Hawa, G., Chapurlat, R., Szulc, P., 2012. The Journal of Clinical Endocrinology & Metabolism 97, E575–E583.
- Serum sclerostin increases in healthy adult men during bed rest.
Spatz, J.M., Fields, E.E., Yu, E.W., Divieti Pajevic, P., Bouxsein, M.L., Sibonga, J.D., Zwart, S.R., Smith, S.M., 2012. J. Clin. Endocrinol. Metab. 97, E1736-1740.
PMID:22767636
- Osteoprotegerin as a predictor of renal and cardiovascular outcomes in renal transplant recipients: follow-up data from the ALERT study.
Svensson, M., Dahle, D.O., Mjøen, G., Weihrauch, G., Scharnagl, H., Dobnig, H., März, W., Jardine, A., Fellström, B., Holdaas, H., 2012. Nephrol. Dial. Transplant. 27, 2571–2575.
PMID:22172725
- Omentin-1, visfatin and adiponectin levels in relation to bone mineral density in Iranian postmenopausal women.
Tohidi, M., Akbarzadeh, S., Larijani, B., Kalantarhormozi, M., Ostovar, A., Assadi, M., Vahdat, K., Farrokhnia, M., Sanjdideh, Z., Amirinejad, R., Nabipour, I., 2012. Bone 51, 876–881.
PMID:22971441
- Serum osteoprotegerin and sex steroid levels in patients with prostate cancer.
Varsavsky, M., Reyes-Garcia, R., Avilés Perez, M.D., Gonzalez Ramírez, A.R., Mijan, J.L., Muñoz-Torres, M., 2012. J. Androl. 33, 594–600.
PMID:21903971
- Vertebral fractures in patients with inflammatory bowel disease compared with a healthy population: a prospective case-control study.
Vázquez, M.A., Lopez, E., Montoya, M.J., Giner, M., Pérez-Temprano, R., Pérez-Cano, R., 2012. BMC Gastroenterol 12, 47. PMCID: PMC3438096
PMID:22584049
- The relationship between osteoclastogenic and anti-osteoclastogenic pro-inflammatory cytokines differs in human osteoporotic and osteoarthritic bone tissues.
Zupan, J., Komadina, R., Marc, J., 2012. J. Biomed. Sci. 19, 28. PMCID: PMC3307025
PMID:22380539
- Correlation of circulating omentin-1 with bone mineral density in multiple sclerosis: the crosstalk between bone and adipose tissue.
Assadi, M., Salimipour, H., Akbarzadeh, S., Nemati, R., Jafari, S.M., Bargahi, A., Samani, Z., Seyedabadi, M., Sanjdideh, Z., Nabipour, I., 2011. PLoS ONE 6, e24240. PMCID: PMC3174149
PMID:21935388
- The RANKL/RANK/OPG signaling pathway mediates medial arterial calcification in diabetic Charcot neuroarthropathy.
Ndip, A., Williams, A., Jude, E.B., Serracino-Inglott, F., Richardson, S., Smyth, J.V., Boulton, A.J.M., Alexander, M.Y., 2011. Diabetes 60, 2187–2196. PMCID: PMC3142088
PMID:21659498
- Bone microstructural changes revealed by high-resolution peripheral quantitative computed tomography imaging and elevated DKK1 and MIP-1 levels in patients with MGUS.
Ng, A.C., Khosla, S., Charatcharoenwitthaya, N., Kumar, S.K., Achenbach, S.J., Holets, M.F., McCready, L.K., Melton, L.J., Kyle, R.A., Rajkumar, S.V., Drake, M.T., 2011. Blood 118, 6529–6534.
- Effect of pioglitazone on serum concentrations of osteoprotegerin in patients with type 2 diabetes mellitus.
Park, J.S., Cho, M.H., Nam, J.S., Yoo, J.S., Ahn, C.W., Cha, B.S., Kim, K.R., Lee, H.C., 2011. Eur. J. Endocrinol. 164, 69–74. PMCID: PMC3000683
PMID:20961967
- Circulating osteoprotegerin and soluble receptor activator of nuclear factor κB ligand in polycystic ovary syndrome: relationships to insulin resistance and endothelial dysfunction.
Pepene, C.E., Ilie, I.R., Marian, I., Duncea, I., 2011. Eur. J. Endocrinol. 164, 61–68.
PMID:20974706
- Dissociation of osteogenic and immunological effects by the selective glucocorticoid receptor agonist, compound A, in human bone marrow stromal cells.
Rauner, M., Goettsch, C., Stein, N., Thiele, S., Bornhaeuser, M., De Bosscher, K., Haegeman, G., Tuckermann, J., Hofbauer, L.C., 2011. Endocrinology 152, 103–112.
PMID:21084452
- Cortical bone status is associated with serum osteoprotegerin concentration in men: the STRAMBO study.
Szulc, P., Hawa, G., Boutroy, S., Vilayphiou, N., Schoppet, M., Chapurlat, R., Hofbauer, L.C., 2011. J. Clin. Endocrinol. Metab. 96, 2216–2226.
PMID:21565793
- Early effects of IL-6 receptor inhibition on bone homeostasis: a pilot study in women with rheumatoid arthritis.
Terpos, E., Fragiadaki, K., Konsta, M., Bratengeier, C., Papatheodorou, A., Sfikakis, P.P., 2011. Clin. Exp. Rheumatol. 29, 921–925
PMID:22032557
- Impact of sirolimus, tacrolimus and mycophenolate mofetil on osteoclastogenesis--implications for post-transplantation bone disease.
Westenfeld, R., Schlieper, G., Wöltje, M., Gawlik, A., Brandenburg, V., Rutkowski, P., Floege, J., Jahnen-Dechent, W., Ketteler, M., 2011. Nephrol. Dial. Transplant. 26, 4115–4123.
PMID:21622987
- Persistent increase of osteoprotegerin levels after cortisol normalization in patients with Cushing’s syndrome.
Camozzi, V., Sanguin, F., Albigier, N., Scaroni, C., Mantero, F., Zaninotto, M., Frigo, A., Piccolo, M., Luisetto, G., 2010. Eur. J. Endocrinol. 162, 85–90.
PMID:19793762
- The Src inhibitor dasatinib accelerates the differentiation of human bone marrow-derived mesenchymal stromal cells into osteoblasts.
Id Boufker, H., Lagneaux, L., Najar, M., Piccart, M., Ghanem, G., Body, J.-J., Journé, F., 2010. BMC Cancer 10, 298. PMCID: PMC3087319
PMID:20565769
- Serum bone turnover markers may be involved in the metastatic potential of lung cancer patients.
Karapanagiotou, E.M., Terpos, E., Dilana, K.D., Alamara, C., Gkiozos, I., Polyzos, A., Syrigos, K.N., 2010. Med. Oncol. 27, 332–338.
PMID:19373566
- Diffuse osteosclerosis complicating hairy cell leukemia
Leung, R., Lopes, D., Lam, C., Wong, K.-F., Kung, A.W.C., Kwong, Y.-L., 2010. J. Clin. Oncol. 28, e203-204.
PMID:20194859
- Biomarkers of the osteoprotegerin pathway: clinical correlates, subclinical disease, incident cardiovascular disease, and mortality.
Lieb, W., Gona, P., Larson, M.G., Massaro, J.M., Lipinska, I., Keaney, J.F., Rong, J., Corey, D., Hoffmann, U., Fox, C.S., Vasan, R.S., Benjamin, E.J., O’Donnell, C.J., Kathiresan, S., 2010. Arterioscler. Thromb. Vasc. Biol. 30, 1849–1854. PMCID: PMC3039214
PMID:20448212
- Effects of pravastatin on serum osteoprotegerin levels in patients with hypercholesterolemia and type 2 diabetes.
Mori, K., Jono, S., Emoto, M., Kawagishi, T., Yasumoto, H., Konishi, T., Furumitsu, Y., Shioi, A., Shoji, T., Inaba, M., Nishizawa, Y., 2010. Angiology 61, 86–91.
PMID:19147525
- Chronic antiepileptic monotherapy, bone metabolism, and body composition in non-institutionalized children.
Rauchenzauner, M., Griesmacher, A., Tatarczyk, T., Haberlandt, E., Strasak, A., Zimmerhackl, L.-B., Falkensammer, G., Luef, G., Högler, W., 2010. Dev Med Child Neurol 52, 283–288.
PMID:19709134
- Biomarkers in early rheumatoid arthritis: longitudinal associations with inflammation and joint destruction measured by magnetic resonance imaging and conventional radiographs.
Syversen, S.W., Haavardsholm, E.A., Bøyesen, P., Goll, G.L., Okkenhaug, C., Gaarder, P.I., van der Heijde, D., Kvien, T.K., 2010. Ann. Rheum. Dis. 69, 845–850.
PMID:20233753
- Baseline RANKL:OPG ratio and markers of bone and cartilage degradation predict annual radiological progression over 11 years in rheumatoid arthritis.
an Tuyl, L.H.D., Voskuyl, A.E., Boers, M., Geusens, P., Landewé, R.B.M., Dijkmans, B.A.C., Lems, W.F., 2010. Ann. Rheum. Dis. 69, 1623–1628.
PMID:20525836
- Serum RANKL, osteoprotegerin (OPG), and RANKL/OPG ratio in nephrotic children.
Wasilewska, A., Rybi-Szuminska, A., Zoch-Zwierz, W., 2010. Pediatr. Nephrol. 25, 2067–2075. PMCID: PMC2923718
PMID:20602239
- Osteoclastogenesis in children with 21-hydroxylase deficiency on long-term glucocorticoid therapy: the role of receptor activator of nuclear factor-kappaB ligand/osteoprotegerin imbalance.
Faienza, M.F., Brunetti, G., Colucci, S., Piacente, L., Ciccarelli, M., Giordani, L., Del Vecchio, G.C., D’Amore, M., Albanese, L., Cavallo, L., Grano, M., 2009. J. Clin. Endocrinol. Metab. 94, 2269–2276.
PMID:19401376
- Bone mineral density and serum levels of soluble tumor necrosis factors, estradiol, and osteoprotegerin in postmenopausal women with cirrhosis after viral hepatitis.
González-Calvin, J.L., Mundi, J.L., Casado-Caballero, F.J., Abadia, A.C., Martin-Ibañez, J.J., 2009. J. Clin. Endocrinol. Metab. 94, 4844–4850.
PMID:19897681
- High osteoprotegerin serum levels in primary biliary cirrhosis are associated with disease severity but not with the mRNA gene expression in liver tissue.
Guañabens, N., Enjuanes, A., Alvarez, L., Peris, P., Caballería, L., Jesús Martínez de Osaba, M., Cerdá, D., Monegal, A., Pons, F., Parés, A., 2009. J. Bone Miner. Metab. 27, 347–354.
PMID:19229472
- Relationships between OPG, RANKL, bone metabolism, and bone mineral density in biliary atresia.
Honsawek, S., Chaiwatanarat, T., Vejchapipat, P., Chongsrisawat, V., Thawornsuk, N., Poovorawan, Y., 2009. Pediatr. Surg. Int. 25, 261–267.
PMID:19184056
- A 246-km continuous running race causes significant changes in bone metabolism.
A 246-km continuous running race causes significant changes in bone metabolism.
PMID:19665602
- Osteoprotegerin levels in women with prior gestational diabetes mellitus.
Madarász, E., Tamás, G., Tabák, A.G., Speer, G., Lakatos, P., Kerényi, Z., 2009. Diabetes Care 32, e5.
PMID:19114622
- Progression of coronary artery calcification in renal transplantation and the role of secondary hyperparathyroidism and inflammation.
Mazzaferro, S., Pasquali, M., Taggi, F., Baldinelli, M., Conte, C., Muci, M.L., Pirozzi, N., Carbone, I., Francone, M., Pugliese, F., 2009. Clin J Am Soc Nephrol 4, 685–690. PMCID: PMC265365
PMID:19211668
- RANKL increases vascular smooth muscle cell calcification through a RANK-BMP4-dependent pathway.
Panizo, S., Cardus, A., Encinas, M., Parisi, E., Valcheva, P., López-Ongil, S., Coll, B., Fernandez, E., Valdivielso, J.M., 2009. Circ. Res. 104, 1041–1048.
PMID:19325147
- The Effect of Zoledronic Acid on Serum Dickkopf-1, Osteoprotegerin, and RANKL in Patients with Paget’s Disease of Bone.
Polyzos, S.A., Anastasilakis, A.D., Efstathiadou, Z., Kita, M., Litsas, I., Avramidis, A., Arsos, G., Moralidis, E., Gerou, S., Pavlidou, V., Papatheodorou, A., Terpos, E., 2009. Hormone and Metabolic Research 41, 846–850.
- The HLA-B27 transgenic rat, a model of spondyloarthritis, has decreased bone mineral density and increased RANKL to osteoprotegerin mRNA ratio.
Rauner, M., Stupphann, D., Haas, M., Fert, I., Glatigny, S., Sipos, W., Breban, M., Pietschmann, P., 2009. J. Rheumatol. 36, 120–126.
PMID:19040304
- Bone turnover and the osteoprotegerin-RANKL pathway in tumor-induced osteomalacia: a longitudinal study of five cases.
Rendina, D., De Filippo, G., Tauchmanovà, L., Insabato, L., Muscariello, R., Gianfrancesco, F., Esposito, T., Cioffi, M., Colao, A., Strazzullo, P., Mossetti, G., 2009. Calcif. Tissue Int. 85, 293–300.
PMID:19763378
- ADA-deficient SCID is associated with a specific microenvironment and bone phenotype characterized by RANKL/OPG imbalance and osteoblast insufficiency.
Sauer, A.V., Mrak, E., Hernandez, R.J., Zacchi, E., Cavani, F., Casiraghi, M., Grunebaum, E., Roifman, C.M., Cervi, M.C., Ambrosi, A., Carlucci, F., Roncarolo, M.G., Villa, A., Rubinacci, A., Aiuti, A., 2009. Blood 114, 3216–3226.
PMID:19633200
- Cartilage and bone biomarkers in rheumatoid arthritis: prediction of 10-year radiographic progression.
Syversen, S.W., Goll, G.L., van der Heijde, D., Landewé, R., Gaarder, P.I., Odegård, S., Haavardsholm, E.A., Kvien, T.K., 2009. J. Rheumatol. 36, 266–272.
PMID:19132792
- High Serum Sclerostin Correlates with Advanced Stage, Increased Bone Resorption, Reduced Osteoblast Function, and Poor Survival in Newly-Diagnosed Patients with Multiple Myeloma.
Terpos, E., Christoulas, D., Katodritou, E., Bratengeier, C., Lindner, B., Harmelin, S., Hawa, G., Boutsikas, G., Migkou, M., Gavriatopoulou, M., 2009a
- The clinical significance of serum markers of bone turnover in NSCLC patients: surveillance, management and prognostic implications.
Terpos, E., Kiagia, M., Karapanagiotou, E.M., Charpidou, A., Dilana, K.D., Nasothimiou, E., Harrington, K.J., Polyzos, A., Syrigos, K.N., 2009b. Anticancer Res. 29, 1651–1657 PMID: 19443381
- Proinflammatory cytokines and receptor activator of nuclear factor kappaB-ligand/osteoprotegerin associated with bone deterioration in patients with Crohn’s disease.
Turk, N., Cukovic-Cavka, S., Korsic, M., Turk, Z., Vucelic, B., 2009. Eur J Gastroenterol Hepatol 21, 159–166.
PMID:19098682
- Increased serum osteoprotegerin levels associated with decreased bone mineral density in familial Mediterranean fever.
Yuksel, S., Samli, H., Colbay, M., Dundar, U., Acarturk, G., Demir, S., Koken, T., Aktepe, O.C., Kavuncu, V., Solak, M., 2009. Tohoku J. Exp. Med. 217, 321–327
PMID:19346738
- Does osteoprotegerin or receptor activator of nuclear factor-kappaB ligand mediate the association between bone and coronary artery calcification?
Bakhireva, L.N., Laughlin, G.A., Bettencourt, R., Barrett-Connor, E., 2008. J. Clin. Endocrinol. Metab. 93, 2009–2012. PMCID: PMC2386279
PMID:18319315
- Risedronate reduces osteoclast precursors and cytokine production in postmenopausal osteoporotic women.
D’Amelio, P., Grimaldi, A., Di Bella, S., Tamone, C., Brianza, S.Z.M., Ravazzoli, M.G.A., Bernabei, P., Cristofaro, M.A., Pescarmona, G.P., Isaia, G., 2008. J. Bone Miner. Res. 23, 373–379.
PMID:17967134
- Constitutional thinness: unusual human phenotype of low bone quality.
Galusca, B., Zouch, M., Germain, N., Bossu, C., Frere, D., Lang, F., Lafage-Proust, M.-H., Thomas, T., Vico, L., Estour, B., 2008. J. Clin. Endocrinol. Metab. 93, 110–117.
PMID:17956951
- Modifying RANKL/OPG mRNA expression in differentiating and growing human primary osteoblasts.
Giner, M., Montoya, M.J., Vázquez, M.A., Rios, M.J., Moruno, R., Miranda, M.J., Pérez-Cano, R., 2008. Horm. Metab. Res. 40, 869–874.
PMID:18932123
- Serum levels of receptor activator of nuclear factor kappaB ligand (RANKL) in healthy women and men.
Kerschan-Schindl, K., Wendlova, J., Kudlacek, S., Gleiss, A., Woloszczuk, W., Pietschmann, P., 2008. Exp. Clin. Endocrinol. Diabetes 116, 491–495.
PMID:18072013
- The differential expression of osteoprotegerin (OPG) and receptor activator of nuclear factor kappaB ligand (RANKL) in human osteoarthritic subchondral bone osteoblasts is an indicator of the metabolic state of these disease cells.
Kwan Tat, S., Pelletier, J.-P., Lajeunesse, D., Fahmi, H., Lavigne, M., Martel-Pelletier, J., 2008. Clin. Exp. Rheumatol. 26, 295–304 PMCID: PMC5247261
PMID:18565252
- Increased augmentation index and central aortic blood pressure in osteoporotic postmenopausal women.
Mangiafico, R.A., Alagona, C., Pennisi, P., Parisi, N., Mangiafico, M., Purrello, F., Fiore, C.E., 2008. Osteoporos Int 19, 49–56.
PMID:17676381
- Osteoclast inhibitory effects of vitamin K2 alone or in combination with etidronate or risedronate in patients with rheumatoid arthritis: 2-year results.
Morishita, M., Nagashima, M., Wauke, K., Takahashi, H., Takenouchi, K., 2008. J. Rheumatol. 35, 407–413
PMID:18260178
- The role of the receptor activator of nuclear factor-kappaB ligand/osteoprotegerin cytokine system in primary hyperparathyroidism.
Nakchbandi, I.A., Lang, R., Kinder, B., Insogna, K.L., 2008. J. Clin. Endocrinol. Metab. 93, 967–973.
PMID:18073309
- High levels of synovial fluid osteoprotegerin (OPG) and increased serum ratio of receptor activator of nuclear factor-kappa B ligand (RANKL) to OPG correlate with disease severity in patients with primary knee osteoarthritis.
Pilichou, A., Papassotiriou, I., Michalakakou, K., Fessatou, S., Fandridis, E., Papachristou, G., Terpos, E., 2008. Clin. Biochem. 41, 746–749.
PMID:18355453
- Increased plasma osteoprotegerin concentrations are associated with indices of bone strength of the hip.
Samelson, E.J., Broe, K.E., Demissie, S., Beck, T.J., Karasik, D., Kathiresan, S., Kiel, D.P., 2008. J. Clin. Endocrinol. Metab. 93, 1789–1795. PMCID: PMC2386280
PMID:18303076
- Bone mineral apparent density in juvenile dermatomyositis: the role of lean body mass and glucocorticoid use.
Santiago, R.A., Silva, C. a. A., Caparbo, V.F., Sallum, A.M.E., Pereira, R.M.R., 2008. Scand. J. Rheumatol. 37, 40–47.
PMID:18189194
- The circulating calcification inhibitors, fetuin-A and osteoprotegerin, but not matrix Gla protein, are associated with vascular stiffness and calcification in children on dialysis.
Shroff, R.C., Shah, V., Hiorns, M.P., Schoppet, M., Hofbauer, L.C., Hawa, G., Schurgers, L.J., Singhal, A., Merryweather, I., Brogan, P., Shanahan, C., Deanfield, J., Rees, L., 2008. Nephrol. Dial. Transplant. 23, 3263–3271.
PMID:18463323
- Osteoprotegerin/RANKL system imbalance in active polyarticular-onset juvenile idiopathic arthritis: a bone damage biomarker?
Spelling, P., Bonfá, E., Caparbo, V.F., Pereira, R.M.R., 2008. Scand. J. Rheumatol. 37, 439–444.
PMID:18802807
- Relationships between insulin-like growth factor-I (IGF-I) and OPG, RANKL, bone mineral density in healthy Chinese women.
Zhao, H.-Y., Liu, J.-M., Ning, G., Zhao, Y.-J., Chen, Y., Sun, L.-H., Zhang, L.-Z., Xu, M.-Y., Chen, J.-L., 2008. Osteoporos Int 19, 221–226.
PMID:17703270
- Determinants of progression of coronary artery calcification in type 2 diabetes role of glycemic control and inflammatory/vascular calcification markers.
Anand, D.V., Lim, E., Darko, D., Bassett, P., Hopkins, D., Lipkin, D., Corder, R., Lahiri, A., 2007. J. Am. Coll. Cardiol. 50, 2218–2225.
PMID:18061069
- Effects on osteoclast and osteoblast activities in cultured mouse calvarial bones by synovial fluids from patients with a loose joint prosthesis and from osteoarthritis patients.
Andersson, M.K., Lundberg, P., Ohlin, A., Perry, M.J., Lie, A., Stark, A., Lerner, U.H., 2007. Arthritis Res. Ther. 9, R18. PMCID: PMC1860076
PMID:17316439
- Circulating osteoprotegerin and receptor activator of NF-kappaB ligand system in patients with beta-thalassemia major.
Angelopoulos, N.G., Goula, A., Katounda, E., Rombopoulos, G., Kaltzidou, V., Kaltsas, D., Malaktari, S., Athanasiou, V., Tolis, G., 2007. J. Bone Miner. Metab. 25, 60–67.
PMID:17187195
- Serum osteoprotegerin concentration with strontium ranelate treatment for postmenopausal osteoporosis: an open, prospective study.
Ertorer, M.E., Bakiner, O., Anaforoglu, I., Sezgin, N., Demirag, N.G., Tutuncu, N.B., 2007. Curr Ther Res Clin Exp 68, 217–225. PMCID: PMC3967269
PMID:24683212
- Serum levels of OPG, RANKL and RANKL/OPG ratio in newly-diagnosed patients with multiple myeloma. Clinical correlations.
Goranova-Marinova, V., Goranov, S., Pavlov, P., Tzvetkova, T., 2007. Haematologica 92, 1000–1001
PMID:17606458
- Increased circulating concentrations and augmented myocardial extraction of osteoprotegerin in heart failure due to left ventricular pressure overload.
Helske, S., Kovanen, P.T., Lindstedt, K.A., Salmela, K., Lommi, J., Turto, H., Werkkala, K., Kupari, M., 2007. Eur. J. Heart Fail. 9, 357–363.
PMID:17254844
- Inflammatory biomarkers are associated with total brain volume: the Framingham Heart Study.
Jefferson, A.L., Massaro, J.M., Wolf, P.A., Seshadri, S., Au, R., Vasan, R.S., Larson, M.G., Meigs, J.B., Keaney, J.F., Lipinska, I., Kathiresan, S., Benjamin, E.J., DeCarli, C., 2007. Neurology 68, 1032–1038. PMCID: PMC2758770
PMID:17389308
- The circadian rhythm of osteoprotegerin and its association with parathyroid hormone secretion.
Joseph, F., Chan, B.Y., Durham, B.H., Ahmad, A.M., Vinjamuri, S., Gallagher, J.A., Vora, J.P., Fraser, W.D., 2007. J. Clin. Endocrinol. Metab. 92, 3230–3238.
PMID:17550963
- NF-kappaB inhibitor dehydroxymethylepoxyquinomicin suppresses osteoclastogenesis and expression of NFATc1 in mouse arthritis without affecting expression of RANKL, osteoprotegerin or macrophage colony-stimulating factor.
Kubota, T., Hoshino, M., Aoki, K., Ohya, K., Komano, Y., Nanki, T., Miyasaka, N., Umezawa, K., 2007. Arthritis Res. Ther. 9, R97. PMCID: PMC2212584
PMID:17892600
- Autoantibodies, metalloproteinases and bone markers in rheumatoid arthritis patients are unable to predict their responses to infliximab.
Lequerré, T., Jouen, F., Brazier, M., Clayssens, S., Klemmer, N., Ménard, J.-F., Mejjad, O., Daragon, A., Tron, F., Le Loët, X., Vittecoq, O., 2007. Rheumatology (Oxford) 46, 446–453.
PMID:16899502
- Increased formation of 8-iso-prostaglandin F(2alpha) is associated with altered bone metabolism and lower bone mass in hypercholesterolaemic subjects.
Mangiafico, R.A., Malaponte, G., Pennisi, P., Li Volti, G., Trovato, G., Mangiafico, M., Bevelacqua, Y., Mazza, F., Fiore, C.E., 2007. J. Intern. Med. 261, 587–596.
PMID:17547714
- Serum OPG and RANKL levels before and after intravenous bisphosphonate treatment in Paget’s disease of bone.
Martini, G., Gennari, L., Merlotti, D., Salvadori, S., Franci, M.B., Campagna, S., Avanzati, A., De Paola, V., Valleggi, F., Nuti, R., 2007. Bone 40, 457–463.
PMID:16979395
- Changes of OPG and RANKL concentrations in Crohn’s disease after infliximab therapy.
Miheller, P., Muzes, G., Rácz, K., Blázovits, A., Lakatos, P., Herszényi, L., Tulassay, Z., 2007. Inflamm. Bowel Dis. 13, 1379–1384.
PMID:17663430
- Serum osteoprotegerin and its ligand in cirrhotic patients referred for orthotopic liver transplantation: relationship with metabolic bone disease.
Monegal, A., Navasa, M., Peris, P., Alvarez, L., Pons, F., Rodés, J., Guañabens, N., 2007. Liver Int. 27, 492–497.
PMID:17403189
- The “lively” cytokines network in beta-Thalassemia Major-related osteoporosis.
Morabito, N., Russo, G.T., Gaudio, A., Lasco, A., Catalano, A., Morini, E., Franchina, F., Maisano, D., La Rosa, M., Plota, M., Crifò, A., Meo, A., Frisina, N., 2007. Bone 40, 1588–1594.
PMID:17412659
- Bone structure and metabolism in a rodent model of male senile osteoporosis.
Pietschmann, P., Skalicky, M., Kneissel, M., Rauner, M., Hofbauer, G., Stupphann, D., Viidik, A., 2007. Exp. Gerontol. 42, 1099–1108.
PMID:17949933
- RANKL:osteoprotegerin ratio and bone mineral density in children with untreated juvenile dermatomyositis.
Rouster-Stevens, K.A., Langman, C.B., Price, H.E., Seshadri, R., Shore, R.M., Abbott, K., Pachman, L.M., 2007. Arthritis Rheum. 56, 977–983.
PMID:17328075
- The sex-specific association of serum osteoprotegerin and receptor activator of nuclear factor kappaB legend with bone mineral density in older adults: the Rancho Bernardo study.
Stern, A., Laughlin, G.A., Bergstrom, J., Barrett-Connor, E., 2007. Eur. J. Endocrinol. 156, 555–562. PMCID: PMC2642656
PMID:17468191
- Elevated soluble receptor activator of nuclear factor-kappaB ligand and reduced bone mineral density in patients with adolescent idiopathic scoliosis.
Suh, K.T., Lee, S.-S., Hwang, S.H., Kim, S.-J., Lee, J.S., 2007. Eur Spine J 16, 1563–1569. PMCID: PMC2078303
PMID:17520299
- Juvenile Paget’s disease: the second reported, oldest patient is homozygous for the TNFRSF11B “Balkan” mutation (966_969delTGACinsCTT), which elevates circulating immunoreactive osteoprotegerin levels.
Whyte, M.P., Singhellakis, P.N., Petersen, M.B., Davies, M., Totty, W.G., Mumm, S., 2007. J. Bone Miner. Res. 22, 938–946.
PMID:17352649
- Association between phosphate removal and markers of bone turnover in haemodialysis patients.
Albalate, M., de la Piedra, C., Fernández, C., Lefort, M., Santana, H., Hernando, P., Hernández, J., Caramelo, C., 2006. Nephrol. Dial. Transplant. 21, 1626–1632.
PMID:16490746
- The relationship between plasma osteoprotegerin levels and coronary artery calcification in uncomplicated type 2 diabetic subjects.
Anand, D.V., Lahiri, A., Lim, E., Hopkins, D., Corder, R., 2006. J. Am. Coll. Cardiol. 47, 1850–1857.
PMID:16682312
- Osteoprotegerin and C-telopeptide of type I collagen in Polish healthy children and adolescents.
Gajewska, J., Ambroszkiewicz, J., Laskowska-Klita, T., 2006. Adv Med Sci 51, 269–272
PMID:17357324
- Influence of Renal Failure on the Osteoprotegerin Level in Patients with Multiple Myeloma.
Goranov, S., Goranova-Marinova, V., Kumchev, E., Pavlov, P., Tzvetkova, T., 2006. Blood 108, 4999–4999
 Download biomedica product list 2018
Download biomedica product list 2018