Periostin Human ELISA
-
Category number
BI-20433
-
Method
Sandwich ELISA, HRPO/TMB, 12×8-well detachable strips
-
Sample type
Serum, EDTA plasma, citrate plasma, heparin plasma
-
Sample volume
10 µl / well
-
Assay time
2 h / 2 h / 1 h / 30 min
-
Sensitivity
20 pmol/l
-
Standard range
0 – 364 000 pg/ml
0 – 4000 pmol/l
-
Conversion factor
1 ng/ml = 0.011 pmol/l (MW: 91 kDa)
-
Regulatory status
Research use only.
Product Overview
The human periostin immunoassay is a 3.5 hour, 96-well sandwich ELISA for the quantitative determination of periostin in human serum and plasma. The assay employs human serum-based standards to ensure the measurement of biologically reliable data. The human periostin kit uses highly purified, epitope mapped antibodies with characterized binding kinetics. The detection antibody used in the human periostin ELISA kit binds to a linear epitope close to the N-terminus in the FAS4 domain of periostin and the polyclonal coating antibody recognizes multiple linear epitopes distributed over the periostin molecule.
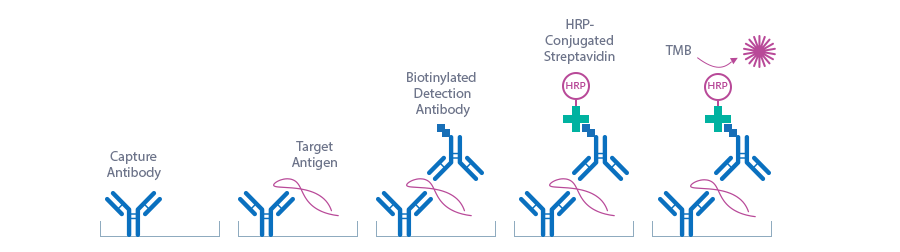
Principle of the Assay
1+50 pre-diluted standard/sample/control and goat biotinylated anti-periostin antibody (AB) are pipetted into the wells of the microtiter strips, which are pre-coated with anti-human periostin antibody. periostin present in the STD/sample/CTRL binds to the pre-coated antibody in the well and forms a sandwich with the detection antibody. In a washing step all non-specific unbound material is removed.
In a second step, the streptavidin-HRPO conjugate is added. After another washing step, substrate (TMB) is pipetted into the wells. The enzyme catalyzed color change of the substrate is directly proportional to the amount of periostin. This color change is detectable with a standard microtiter plate ELISA reader. A dose response curve of the absorbance (optical density, OD at 450 nm) vs. standard concentration is generated, using the values obtained from the standard. The concentration of human periostin in the sample is determined directly from the dose response curve.
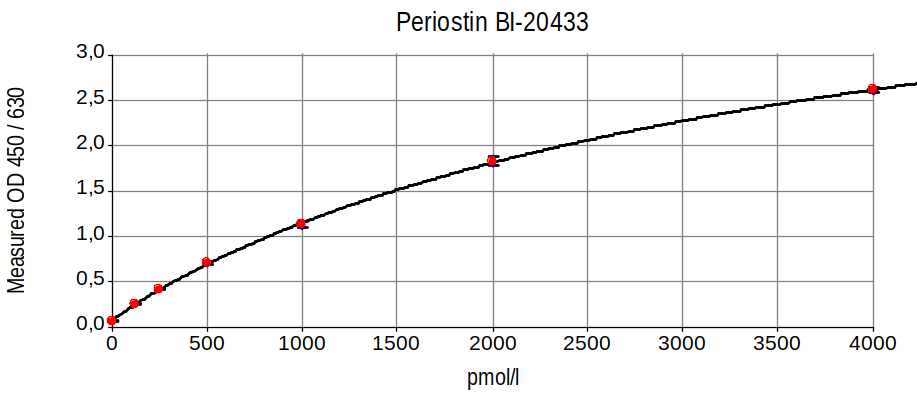
Typical Standard Curve
The figure below shows a typical standard curve for the human periostin ELISA. The immunoassay is calibrated against recombinant periostin peptide:
Kit components
| CONT | KIT COMPONENTS | QUANTITY |
| PLATE | Mouse monoclonal anti-human periostin antibody, pre-coated microtiter strips in a stripholder, packed in an aluminium bag with desiccant | 12 x 8 tests |
| WASHBUF | Wash buffer concentrate 20x, natural cap | 1 x 50 ml |
| AB | Goat polyclonal anti-human periostin antibody, biotinylated, green cap, ready to use | 1 x 18 ml |
| STD | Standards 1-7, (0, 125, 250, 500, 1000, 2000, 4000 pmol/l), white caps , lyophilised | 7 vials |
| CTRL | Control A and B, yellow cap, lyophilised (for concentration see label) | 2 vials |
| ASYBUF | Assay Buffer, red cap, ready to use | 1 x 55 ml |
| CONJ | Conjugate, (streptavidin-HRPO), amber bottle, amber cap, ready to use | 1 x 18 ml |
| SUB | Substrate (TMB solution), amber bottle, blue cap, ready to use | 1 x 22 ml |
| STOP | Stop solution, white cap, ready to use | 1 x 7 ml |
Storage instructions: All reagents of the human periostin ELISA kit are stable at 4°C until the expiry date stated on the label of each reagent.
Sample Collection & Storage
Serum and plasma are suitable for use in this assay. We recommend duplicate measurements for all samples, standards and controls. Do not change sample type during studies.
Collect venous blood samples by using standardized blood collection tubes for serum or plasma. Perform serum and plasma separation by centrifugation according to supplier’s instructions of the blood collection devices and measure the acquired serum or plasma samples as soon as possible. For longer storage aliquot and store at -25°C or lower. Samples are stable for 4 freeze-thaw cycles. Thawed samples should be assayed as soon as possible. Lipemic or haemolysed samples may give erroneous results. Samples should be mixed well before assaying.
Reagent Preparation
Wash Buffer
|
1. |
Bring the WASHBUF concentrate to room temperature (18-26°C). Crystals in the buffer concentrate will dissolve at room temperature. |
|
2. |
Dilute the WASHBUF concentrate 1:20, e.g., 50ml WASHBUF + 950ml distilled or deionized water. Only use diluted WASHBUF when performing the assay. |
The diluted WASHBUF is stable up to one month at 4°C (2-8°C).
Sample Preparation
Bring samples to room temperature and mix samples gently to ensure the samples are homogenous. We recommend duplicate measurements for all samples.
SERUM or PLASMA Samples (human or non-human samples) and CELL CULTURE SUPERNATANTS must be diluted 1+50 with ASYBUF (assay buffer) prior to the assay, e.g. 10 µl sample + 500 µl ASYBUF. Note: 150 µl pre-diluted sample is required / well.
URINE Samples must be diluted at least 1+3 with ASYBUF (assaybuffer) prior to the assay, e.g. 100 µl sample + 300 µl ASYBUF. Note: 150 µl pre-diluted sample is required / well.
Assay Protocol
Read the entire protocol before beginning the assay.
|
1. |
Mark positions for STD/SAMPLE/CTRL (Standard/Sample/Control) on the protocol sheet. |
|
2. |
Take microtiter strips out of the aluminum bag. Store unused strips with desiccant at 4°C in the aluminum bag. Strips are stable until expiry date stated on the label. |
|
3. |
Add 150 µl pre-diluted (1+50)* STD/CTRL/SAMPLE into the respective wells. |
|
4. |
Cover the plate tightly and incubate for 2 hours at room temperature (18-26°C). |
|
5. |
Aspirate and wash wells 5 x with 300 µl diluted WASHBUF. After the final wash, remove the remaining WASHBUF by strongly tapping plate against a paper towel. |
|
6. |
Add 150 µl AB (biotinylated anti Periostin antibody, green cap) into each well, swirl gently. |
|
7. |
Cover tightly and incubate for 2 hours at room temperature. |
|
8. |
Aspirate and wash wells 5 x with 300 µl diluted WASHBUF. After the final wash, remove the remaining WASHBUF by strongly tapping plate against a paper towel. |
|
9. |
Add 150 µl CONJ (Conjugate, amber cap) into each well. Swirl gently. |
|
10. |
Cover tightly and incubate for 1 hour at room temperature. |
|
11. |
Aspirate and wash wells 5 x with 300 µl diluted WASHBUF. After the final wash, remove the remaining WASHBUF by strongly tapping plate against a paper towel. |
|
12. |
Add 150 µl SUB (substrate, blue cap) into each well. |
|
13. |
Incubate for 30 min at room temperature in the dark. |
|
14. |
Add 50 µl STOP (stop solution, white cap) into each well. Swirl gently. |
|
15. |
Measure absorbance immediately at 450 nm with reference 630 nm, if available. |
Calculation of Results
Read the optical density (OD) of all wells on a plate reader using 450 nm wavelength (correction wavelength 630 nm). Construct the standard curve from the OD values of the STD. Use commercially available software or graph paper. Obtain sample concentration from this standard curve. The assay was evaluated with 4PL algorithm. Different curve fitting methods need to be evaluated by the user. Sample dilutions above 1+50 have to be considered when calculating the final sample concentration.
The quality control (QC) protocol supplied with the kit shows the results of the final release QC for each kit lot at production date. Data for OD obtained by customers may differ due to various influences and/or due to the normal decrease of signal intensity during shelf life. However, this does not affect validity of results as long as an OD of 1.50 or higher is obtained for the standard with the highest concentration and the values of the CTRLs are in range (target ranges see labels).
Periostin Protein
Periostin (OSF-2) is secreted as a 91 kDa homodimeric soluble extracellular matrix protein expressed in collagen-rich fibrous connective tissues. There are at least 7 isoforms of Periostin, caused by alternative splicing (http://www.uniprot.org/uniprot/Q15063).
|
Molecular Weight |
91 kDa |
|
Cellular localisation |
Extracellular or secreted |
|
Post-translational modifications |
Glycosylation, disulphide bonds |
|
Sequence similarities |
FAS1 superfamily |
|
Alternative Names |
POSTN, OSF-2, OSF2, PDLPN, PN |
|
Pubchem ID |
187888 link: https://pubchem.ncbi.nlm.nih.gov/compound/periostin |
|
Genecards |
POSTN link: https://www.genecards.org/cgi-bin/carddisp.pl?gene=POSTN&keywords=periostin |
|
OMIM |
608777 link: https://www.omim.org/entry/608777 |
|
PDB |
5WT7 link: http://www.rcsb.org/structure/5WT7 |
|
Pfam |
PF02469 link: http://pfam.xfam.org/family/PF02469 |
|
Protein Atlas |
POSTN link: https://www.proteinatlas.org/ENSG00000133110-POSTN/tissue |
|
Uniport ID |
Q15063 link: https://www.uniprot.org/uniprot/Q15063 |
Periostin Function
Periostin is involved in osteoblast recruitment, attachment and spreading. It has been associated with the epithelial-mesenchymal transition in cancer and with the differentiation of mesenchyme in the developing heart. Periostin has functions in osteology, tissue repair, oncology, cardiovascular and respiratory diseases, and in various inflammatory settings.
-
Metabolic Disease
-
Diabetic retinopathy (Ding et al., 2018)
-
-
Nephrology
-
Kidney fibrosis (An et al., 2018; Hwang et al., 2017; Mael-Ainin et al., 2014; Sen et al., 2011)
-
Polycystsic kidney disease (Raman et al., 2018; Wallace et al., 2014)
-
Hypertensive nephropathy (Guerrot et al., 2012)
-
Kidney injury (Satirapoj et al., 2015, 2014, 2012)
-
IgA Nephrophathy (Hwang et al., 2016; Satirapoj et al., 2014)
-
-
Oncology
-
Multiple myeloma (Terpos et al., 2016)
-
Ameloblastoma (Kang et al., 2018)
-
Ovarian cancer (Ryner et al., 2015; Sung et al., 2016; Tang et al., 2018)
-
Papillary thyroid carcinoma (Giusca et al., 2017)
-
Head and neck cancer (Liu et al., 2018)
-
Breast carcinoma (Kim et al., 2017; Lambert et al., 2016; Nuzzo et al., 2016; Ratajczak-Wielgomas et al., 2017)
-
Lung cancer (Che et al., 2017; Zhang et al., 2017)
-
Osteosarcoma (Hu et al., 2016)
-
Gastric cancer (Liu et al., 2016)
-
Bladder cancer (Silvers et al., 2016)
-
Prostate cancer (Tian et al., 2015)
-
-
Osteology
-
Osteoporosis (Kim et al., 2015; Rousseau et al., 2014; Varughese et al., 2018; Yan et al., 2017)
-
Paget’s disease (Polyzos et al., 2017)
-
Osteoarthritis (Chijimatsu et al., 2015; Honsawek et al., 2015b; Rousseau et al., 2014; Tajika et al., 2017)
-
Rheumatoid arthritis (Kerschan-Schindl et al., 2018; Sakellariou et al., 2015)
-
Anyklosing spondylitis (Solmaz u. a., 2018)
-
-
Other
-
Wound healing
-
Miscarriage (Freis et al., 2017)
-
Skin diseases (Murota et al., 2017; Sung et al., 2017)
-
Liver stiffness (Honsawek et al., 2015)
-
-
Respitatory disease
-
Asthma (Carpagnano et al., 2018; Górska et al., 2016; Hoshino et al., 2016; Inoue et al., 2016; Kim et al., 2014; Lee et al., 2018; Matsusaka et al., 2015; O’Dwyer and Moore, 2017; Song et al., 2015)
-
Allergy (Fujishima et al., 2016; Izuhara et al., 2017)
-
Pulmonary fibrosis (Ohta et al., 2017)
-
Bronchopulmoany dysplasia (Ahlfeld et al., 2016)
-
Periostin Induces Kidney Fibrosis after Acute Kidney Injury via the p38 MAPK Pathway.
An, J.N., Yang, S.H., Kim, Y.C., Hwang, J.H., Park, J.Y., Kim, D.K., Kim, J.H., Kim, D.W., Hur, D.G., Oh, Y.K., Lim, C.S., Kim, Y.S., Lee, J.P., 2018. Am. J. Physiol. Renal Physiol.
https://doi.org/10.1152/ajprenal.00203.2018
PMID: 30539653
Looking for Airways Periostin in Severe Asthma: Could It Be Useful for Clustering Type 2 Endotype?
Carpagnano, G.E., Scioscia, G., Lacedonia, D., Soccio, P., Lepore, G., Saetta, M., Foschino Barbaro, M.P., Barnes, P.J., 2018. Chest 154, 1083–1090.
https://doi.org/10.1016/j.chest.2018.08.1032
PMID: 30336944
Increased serum periostin concentrations are associated with the presence of diabetic retinopathy in patients with type 2 diabetes mellitus.
Ding, Y., Ge, Q., Qu, H., Feng, Z., Long, J., Wei, Q., Zhou, Q., Wu, R., Yao, L., Deng, H., 2018. J. Endocrinol. Invest. 41, 937–945.
https://doi.org/10.1007/s40618-017-0820-x
PMID: 29349642
Upregulation of Periostin expression in the pathogenesis of ameloblastoma.
Kang, Y., Liu, J., Zhang, Y., Sun, Y., Wang, J., Huang, B., Zhong, M., 2018. Pathol. Res. Pract. 214, 1959–1965.
https://doi.org/10.1016/j.prp.2018.08.028
PMID: 30196986
Rheumatoid arthritis in remission : Decreased myostatin and increased serum levels of periostin.
Kerschan-Schindl, K., Ebenbichler, G., Föeger-Samwald, U., Leiss, H., Gesslbauer, C., Herceg, M., Stummvoll, G., Marculescu, R., Crevenna, R., Pietschmann, P., 2018. Wien. Klin. Wochenschr.
https://doi.org/10.1007/s00508-018-1386-0
PMID: 30171335
Serum Periostin Levels: A Potential Serologic Marker for Toluene Diisocyanate-Induced Occupational Asthma.
Lee, J.H., Kim, S.H., Choi, Y., Trinh, H.K.T., Yang, E.M., Ban, G.Y., Shin, Y.S., Ye, Y.M., Izuhara, K., Park, H.S., 2018. Yonsei Med. J. 59, 1214–1221.
https://doi.org/10.3349/ymj.2018.59.10.1214
PMID: 30450856; PMCID: PMC6240562
Bone marrow mesenchymal stem cells promote head and neck cancer progression through Periostin-mediated phosphoinositide 3-kinase/Akt/mammalian target of rapamycin.
Liu, C., Feng, X., Wang, B., Wang, X., Wang, C., Yu, M., Cao, G., Wang, H., 2018. Cancer Sci. 109, 688–698.
https://doi.org/10.1111/cas.13479
PMID: 29284199; PMCID: PMC5834805
Periostin overexpression in collecting ducts accelerates renal cyst growth and fibrosis in polycystic kidney disease.
Raman, A., Parnell, S.C., Zhang, Y., Reif, G.A., Dai, Y., Khanna, A., Daniel, E., White, C., Vivian, J.L., Wallace, D.P., 2018. Am. J. Physiol. Renal Physiol.
https://doi.org/10.1152/ajprenal.00246.2018
PMID: 30332313
Cross-talk between ovarian cancer cells and macrophages through periostin promotes macrophage recruitment.
Tang, M., Liu, B., Bu, X., Zhao, P., 2018. Cancer Sci. 109, 1309–1318.
https://doi.org/10.1111/cas.13567
PMID: 29527764; PMCID: PMC5980394
Serum periostin levels following small bone fractures, long bone fractures and joint replacements: an observational study.
Varughese, R., Semprini, R., Munro, C., Fingleton, J., Holweg, C., Weatherall, M., Beasley, R., Braithwaite, I., 2018. Allergy Asthma Clin. Immunol. Off. J. Can. Soc. Allergy Clin. Immunol. 14.
https://doi.org/10.1186/s13223-018-0254-9
PMID: 30065761; PMCID: PMC6060508
Effects of lentivirus-mediated silencing of Periostin on tumor microenvironment and bone metastasis via the integrin-signaling pathway in lung cancer.
Che, J., Shen, W.-Z., Deng, Y., Dai, Y.-H., Liao, Y.-D., Yuan, X.-L., Zhang, P., 2017. Life Sci. 182, 10–21.
https://doi.org/10.1016/j.lfs.2017.05.030
PMID: 28601389
Serum periostin levels in early in pregnancy are significantly altered in women with miscarriage.
Freis, A., Schlegel, J., Kuon, R.J., Doster, A., Jauckus, J., Strowitzki, T., Germeyer, A., 2017. Reprod. Biol. Endocrinol. RBE 15.
https://doi.org/10.1186/s12958-017-0307-9
PMID: 29096644; PMCID: PMC5667517
Heterogeneous Periostin Expression in Different Histological Variants of Papillary Thyroid Carcinoma.
Giusca, S.E., Amalinei, C., Lozneanu, L., Ciobanu Apostol, D., Andriescu, E.C., Scripcariu, A., Balan, R., Avadanei, E.R., Căruntu, I.-D., 2017. BioMed Res. Int. 2017, 8701386.
https://doi.org/10.1155/2017/8701386
PMID: 29435461; PMCID: PMC5757104
Experimental Inhibition of Periostin Attenuates Kidney Fibrosis.
Hwang, J.H., Yang, S.H., Kim, Y.C., Kim, J.H., An, J.N., Moon, K.C., Oh, Y.K., Park, J.Y., Kim, D.K., Kim, Y.S., Lim, C.S., Lee, J.P., 2017. Am. J. Nephrol. 46, 501–517.
https://doi.org/10.1159/000485325
PMID: 29268247
Periostin in inflammation and allergy.
Izuhara, K., Nunomura, S., Nanri, Y., Ogawa, M., Ono, J., Mitamura, Y., Yoshihara, T., 2017. Cell. Mol. Life Sci. CMLS 74, 4293–4303.
https://doi.org/10.1007/s00018-017-2648-0
PMID: 28887633
Epithelial periostin expression is correlated with poor survival in patients with invasive breast carcinoma.
Kim, G.-E., Lee, J.S., Park, M.H., Yoon, J.H., 2017. PloS One 12, e0187635.
https://doi.org/10.1371/journal.pone.0187635
PMID: 29161296; PMCID: PMC5697858
Periostin in the pathogenesis of skin diseases.
Murota, H., Lingli, Y., Katayama, I., 2017. Cell. Mol. Life Sci. CMLS 74, 4321–4328.
https://doi.org/10.1007/s00018-017-2647-1
PMID: 28916993
The role of periostin in lung fibrosis and airway remodeling.
O’Dwyer, D.N., Moore, B.B., 2017. Cell. Mol. Life Sci. CMLS 74, 4305–4314.
https://doi.org/10.1007/s00018-017-2649-z
PMID: 28918442; PMCID: PMC5659879
The usefulness of monomeric periostin as a biomarker for idiopathic pulmonary fibrosis.
Ohta, S., Okamoto, M., Fujimoto, K., Sakamoto, N., Takahashi, K., Yamamoto, H., Kushima, H., Ishii, H., Akasaka, K., Ono, J., Kamei, A., Azuma, Y., Matsumoto, H., Yamaguchi, Y., Aihara, M., Johkoh, T., Kawaguchi, A., Ichiki, M., Sagara, H., Kadota, J.-I., Hanaoka, M., Hayashi, S.-I., Kohno, S., Hoshino, T., Izuhara, K., Consortium for Development of Diagnostics for Pulmonary Fibrosis Patients (CoDD-PF), 2017. PloS One 12, e0174547.
https://doi.org/10.1371/journal.pone.0174547
PMID: 28355256; PMCID: PMC5371347
Periostin and sclerostin levels in juvenile Paget’s disease.
Polyzos, S.A., Makras, P., Anastasilakis, A.D., Mintziori, G., Kita, M., Papatheodorou, A., Kokkoris, P., Terpos, E., 2017. Clin. Cases Miner. Bone Metab. Off. J. Ital. Soc. Osteoporos. Miner. Metab. Skelet. Dis. 14, 269–271.
https://doi.org/10.11138/ccmbm/2017.14.2.269
PMID: 29263750; PMCID: PMC5726226
Expression of periostin in breast cancer cells.
Ratajczak-Wielgomas, K., Grzegrzolka, J., Piotrowska, A., Matkowski, R., Wojnar, A., Rys, J., Ugorski, M., Dziegiel, P., 2017. Int. J. Oncol. 51, 1300–1310.
https://doi.org/10.3892/ijo.2017.4109
PMID: 28902360
An association of periostin levels with the severity and chronicity of atopic dermatitis in children.
Sung, M., Lee, K.S., Ha, E.G., Lee, S.J., Kim, M.A., Lee, S.W., Jee, H.M., Sheen, Y.H., Jung, Y.H., Han, M.Y., 2017. Pediatr. Allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol. 28, 543–550.
https://doi.org/10.1111/pai.12744
PMID: 28631851
Influence of Periostin on Synoviocytes in Knee Osteoarthritis.
Tajika, Y., Moue, T., Ishikawa, S., Asano, K., Okumo, T., Takagi, H., Hisamitsu, T., 2017. Vivo Athens Greece 31, 69–77.
https://doi.org/10.21873/invivo.11027
PMID: 28064223; PMCID: PMC5354150
Circulating periostin levels increase in association with bone density loss and healing progression during the early phase of hip fracture in Chinese older women.
Yan, J., Liu, H.J., Li, H., Chen, L., Bian, Y.Q., Zhao, B., Han, H.X., Han, S.Z., Han, L.R., Wang, D.W., Yang, X.F., 2017. Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 28, 2335–2341.
https://doi.org/10.1007/s00198-017-4034-z
PMID: 28382553
Predictive and prognostic value of serum periostin in advanced non-small cell lung cancer patients receiving chemotherapy.
Zhang, Y., Yuan, D., Yao, Y., Sun, W., Shi, Y., Su, X., 2017. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 39, 1010428317698367.
https://doi.org/10.1177/1010428317698367
PMID: 28459197
Early Elevation of Plasma Periostin Is Associated with Chronic Ventilator-Dependent Bronchopulmonary Dysplasia.
Ahlfeld, S.K., Davis, S.D., Kelley, K.J., Poindexter, B.B., 2016. Am. J. Respir. Crit. Care Med. 194, 1430–1433.
https://doi.org/10.1164/rccm.201605-0910LE
PMID: 27905846; PMCID: PMC5148145
The usefulness of measuring tear periostin for the diagnosis and management of ocular allergic diseases.
Fujishima, H., Okada, N., Matsumoto, K., Fukagawa, K., Igarashi, A., Matsuda, A., Ono, J., Ohta, S., Mukai, H., Yoshikawa, M., Izuhara, K., 2016. J. Allergy Clin. Immunol. 138, 459-467.e2.
https://doi.org/10.1016/j.jaci.2015.11.039
PMID: 26964692
Comparative study of periostin expression in different respiratory samples in patients with asthma and chronic obstructive pulmonary disease.
Górska, K., Maskey-Warzęchowska, M., Nejman-Gryz, P., Korczyński, P., Prochorec-Sobieszek, M., Krenke, R., 2016. Pol. Arch. Med. Wewn. 126, 124–137.
https://doi.org/10.20452/pamw.3299
PMID: 26895432
Association of airway wall thickness with serum periostin in steroid-naive asthma.
Hoshino, M., Ohtawa, J., Akitsu, K., 2016. Allergy Asthma Proc. 37, 225–230.
https://doi.org/10.2500/aap.2016.37.3945
PMID: 27178891
High-level expression of periostin is significantly correlated with tumour angiogenesis and poor prognosis in osteosarcoma.
Hu, F., Shang, X.-F., Wang, W., Jiang, W., Fang, C., Tan, D., Zhou, H.-C., 2016. Int. J. Exp. Pathol. 97, 86–92.
https://doi.org/10.1111/iep.12171
PMID: 27028305; PMCID: PMC4840243
Urinary Periostin Excretion Predicts Renal Outcome in IgA Nephropathy.
Hwang, J.H., Lee, J.P., Kim, C.T., Yang, S.H., Kim, J.H., An, J.N., Moon, K.C., Lee, H., Oh, Y.K., Joo, K.W., Kim, D.K., Kim, Y.S., Lim, C.S., 2016. Am. J. Nephrol. 44, 481–492.
https://doi.org/10.1159/000452228
PMID: 27802442
Periostin as a biomarker for the diagnosis of pediatric asthma.
Inoue, T., Akashi, K., Watanabe, M., Ikeda, Y., Ashizuka, S., Motoki, T., Suzuki, R., Sagara, N., Yanagida, N., Sato, S., Ebisawa, M., Ohta, S., Ono, J., Izuhara, K., Katsunuma, T., 2016. Pediatr. Allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol. 27, 521–526.
https://doi.org/10.1111/pai.12575
PMID: 27062336
Tumor Cell-Derived Periostin Regulates Cytokines That Maintain Breast Cancer Stem Cells.
Lambert, A.W., Wong, C.K., Ozturk, S., Papageorgis, P., Raghunathan, R., Alekseyev, Y., Gower, A.C., Reinhard, B.M., Abdolmaleky, H.M., Thiagalingam, S., 2016. Mol. Cancer Res. MCR 14, 103–113.
https://doi.org/10.1158/1541-7786.MCR-15-0079
PMID: 26507575; PMCID: PMC4715959
Isoprenaline Induces Periostin Expression in Gastric Cancer.
Liu, G.-X., Xi, H.-Q., Sun, X.-Y., Geng, Z.-J., Yang, S.-W., Lu, Y.-J., Wei, B., Chen, L., 2016. Yonsei Med. J. 57, 557–564.
https://doi.org/10.3349/ymj.2016.57.3.557
PMID: 26996552; PMCID: PMC4800342
The prognostic value of stromal and epithelial periostin expression in human breast cancer: correlation with clinical pathological features and mortality outcome.
Nuzzo, P.V., Rubagotti, A., Zinoli, L., Salvi, S., Boccardo, S., Boccardo, F., 2016. BMC Cancer 16, 95.
https://doi.org/10.1186/s12885-016-2139-y
PMID: 26872609; PMCID: PMC4752779
Identification of extracellular vesicle-borne periostin as a feature of muscle-invasive bladder cancer.
Silvers, C.R., Liu, Y.-R., Wu, C.-H., Miyamoto, H., Messing, E.M., Lee, Y.-F., 2016. Oncotarget 7, 23335–23345.
https://doi.org/10.18632/oncotarget.8024
PMID: 26981774; PMCID: PMC5029630
Periostin in tumor microenvironment is associated with poor prognosis and platinum resistance in epithelial ovarian carcinoma.
Sung, P.-L., Jan, Y.-H., Lin, S.-C., Huang, C.-C., Lin, H., Wen, K.-C., Chao, K.-C., Lai, C.-R., Wang, P.-H., Chuang, C.-M., Wu, H.-H., Twu, N.-F., Yen, M.-S., Hsiao, M., Huang, C.-Y.F., 2016. Oncotarget 7, 4036–4047.
https://doi.org/10.18632/oncotarget.6700
PMID: 26716408; PMCID: PMC4826188
High levels of periostin correlate with increased fracture rate, diffuse MRI pattern, abnormal bone remodeling and advanced disease stage in patients with newly diagnosed symptomatic multiple myeloma.
Terpos, E., Christoulas, D., Kastritis, E., Bagratuni, T., Gavriatopoulou, M., Roussou, M., Papatheodorou, A., Eleutherakis-Papaiakovou, E., Kanellias, N., Liakou, C., Panagiotidis, I., Migkou, M., Kokkoris, P., Moulopoulos, L.A., Dimopoulos, M.A., 2016. Blood Cancer J. 6, e482.
https://doi.org/10.1038/bcj.2016.90
PMID: 27716740; PMCID: PMC5098262
Expression and pathological effects of periostin in human osteoarthritis cartilage.
Chijimatsu, R., Kunugiza, Y., Taniyama, Y., Nakamura, N., Tomita, T., Yoshikawa, H., 2015. BMC Musculoskelet. Disord. 16, 215.
https://doi.org/10.1186/s12891-015-0682-3
PMID: 26289167; PMCID: PMC4545863
Elevated serum periostin is associated with liver stiffness and clinical outcome in biliary atresia.
Honsawek, S., Udomsinprasert, W., Vejchapipat, P., Chongsrisawat, V., Phavichitr, N., Poovorawan, Y., 2015a. Biomark. Biochem. Indic. Expo. Response Susceptibility Chem. 20, 157–161.
https://doi.org/10.3109/1354750X.2015.1045032
PMID: 25980529
Association of plasma and synovial fluid periostin with radiographic knee osteoarthritis: Cross-sectional study.
Honsawek, S., Wilairatana, V., Udomsinprasert, W., Sinlapavilawan, P., Jirathanathornnukul, N., 2015b. Jt. Bone Spine Rev. Rhum. 82, 352–355.
https://doi.org/10.1016/j.jbspin.2015.01.023
PMID: 25881760
Plasma periostin associates significantly with non-vertebral but not vertebral fractures in postmenopausal women: Clinical evidence for the different effects of periostin depending on the skeletal site. Kim, B.-J., Rhee, Y., Kim, C.H., Baek, K.H., Min, Y.-K., Kim, D.-Y., Ahn, S.H., Kim, H., Lee, S.H., Lee, S.-Y., Kang, M.-I., Koh, J.-M., 2015. Bone 81, 435–441.
https://doi.org/10.1016/j.bone.2015.08.014
PMID: 26297442
Phenotype of asthma related with high serum periostin levels.
Matsusaka, M., Kabata, H., Fukunaga, K., Suzuki, Y., Masaki, K., Mochimaru, T., Sakamaki, F., Oyamada, Y., Inoue, T., Oguma, T., Sayama, K., Koh, H., Nakamura, M., Umeda, A., Ono, J., Ohta, S., Izuhara, K., Asano, K., Betsuyaku, T., 2015. Allergol. Int. Off. J. Jpn. Soc. Allergol. 64, 175–180.
https://doi.org/10.1016/j.alit.2014.07.003
PMID: 25838094
Upregulation of Periostin and Reactive Stroma Is Associated with Primary Chemoresistance and Predicts Clinical Outcomes in Epithelial Ovarian Cancer.
Ryner, L., Guan, Y., Firestein, R., Xiao, Y., Choi, Y., Rabe, C., Lu, S., Fuentes, E., Huw, L.-Y., Lackner, M.R., Fu, L., Amler, L.C., Bais, C., Wang, Y., 2015. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 21, 2941–2951.
https://doi.org/10.1158/1078-0432.CCR-14-3111
PMID: 25838397
Circulating periostin levels in patients with AS: association with clinical and radiographic variables, inflammatory markers and molecules involved in bone formation.
Sakellariou, G.T., Anastasilakis, A.D., Bisbinas, I., Oikonomou, D., Gerou, S., Polyzos, S.A., Sayegh, F.E., 2015. Rheumatol. Oxf. Engl. 54, 908–914.
https://doi.org/10.1093/rheumatology/keu425
PMID: 25349442
Periostin as a tissue and urinary biomarker of renal injury in type 2 diabetes mellitus.
Satirapoj, B., Tassanasorn, S., Charoenpitakchai, M., Supasyndh, O., 2015. PloS One 10, e0124055.
https://doi.org/10.1371/journal.pone.0124055
PMID: 25884625; PMCID: PMC4401767
Serum periostin levels correlate with airway hyper-responsiveness to methacholine and mannitol in children with asthma.
Song, J.-S., You, J.-S., Jeong, S.-I., Yang, S., Hwang, I.-T., Im, Y.-G., Baek, H.-S., Kim, H.-Y., Suh, D.-I., Lee, H.-B., Izuhara, K., 2015. Allergy 70, 674–681.
https://doi.org/10.1111/all.12599
PMID: 25703927
Overexpression of periostin in stroma positively associated with aggressive prostate cancer.
Tian, Y., Choi, C.H., Li, Q.K., Rahmatpanah, F.B., Chen, X., Kim, S.R., Veltri, R., Chia, D., Zhang, Z., Mercola, D., Zhang, H., 2015. PloS One 10, e0121502.
https://doi.org/10.1371/journal.pone.0121502
PMID: 25781169; PMCID: PMC4362940
Association of serum periostin with aspirin-exacerbated respiratory disease.
Kim, M.-A., Izuhara, K., Ohta, S., Ono, J., Yoon, M.K., Ban, G.Y., Yoo, H.-S., Shin, Y.S., Ye, Y.-M., Nahm, D.-H., Park, H.-S., 2014. Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol. 113, 314–320.
https://doi.org/10.1016/j.anai.2014.06.014
PMID: 25037608
Inhibition of Periostin Expression Protects against the Development of Renal Inflammation and Fibrosis.
Mael-Ainin, M., Abed, A., Conway, S.J., Dussaule, J.-C., Chatziantoniou, C., 2014. J. Am. Soc. Nephrol. 25, 1724–1736.
https://doi.org/10.1681/ASN.2013060664
PMID: 24578131
Serum periostin is associated with fracture risk in postmenopausal women: a 7-year prospective analysis of the OFELY study.
Rousseau, J.C., Sornay-Rendu, E., Bertholon, C., Chapurlat, R., Garnero, P., 2014. J. Clin. Endocrinol. Metab. 99, 2533–2539.
https://doi.org/10.1210/jc.2013-3893
PMID: 24628551
Urine Periostin as a Biomarker of Renal Injury in Chronic Allograft Nephropathy.
Satirapoj, B., Witoon, R., Ruangkanchanasetr, P., Wantanasiri, P., Charoenpitakchai, M., Choovichian, P., 2014. Transplant. Proc. 46, 135–140.
https://doi.org/10.1016/j.transproceed.2013.07.069
Periostin promotes renal cyst growth and interstitial fibrosis in polycystic kidney disease.
Wallace, D.P., White, C., Savinkova, L., Nivens, E., Reif, G.A., Pinto, C.S., Raman, A., Parnell, S.C., Conway, S.J., Fields, T.A., 2014. Kidney Int. 85, 845–854.
https://doi.org/10.1038/ki.2013.488
PMID: 24284511
Identification of periostin as a critical marker of progression/reversal of hypertensive nephropathy.
Guerrot, D., Dussaule, J.-C., Mael-Ainin, M., Xu-Dubois, Y.-C., Rondeau, E., Chatziantoniou, C., Placier, S., 2012. PloS One 7, e31974.
https://doi.org/10.1371/journal.pone.0031974
PMID: 22403621; PMCID: PMC3293874
Periostin: novel tissue and urinary biomarker of progressive renal injury induces a coordinated mesenchymal phenotype in tubular cells.
Satirapoj, B., Wang, Y., Chamberlin, M.P., Dai, T., LaPage, J., Phillips, L., Nast, C.C., Adler, S.G., 2012. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. - Eur. Ren. Assoc. 27, 2702–2711.
https://doi.org/10.1093/ndt/gfr670
PMID: 22167593; PMCID: PMC3471549
Periostin Is Induced in Glomerular Injury and Expressed de Novo in Interstitial Renal Fibrosis.
Sen, K., Lindenmeyer, M.T., Gaspert, A., Eichinger, F., Neusser, M.A., Kretzler, M., Segerer, S., Cohen, C.D., 2011. Am. J. Pathol. 179, 1756–1767.
https://doi.org/10.1016/j.ajpath.2011.06.002
PMID: 21854746
All Biomedica ELISAs are validated according to international FDA/ICH/EMEA guidelines. For more information about our validation guidelines, please refer to our quality page and published validation guidelines and literature.
1. ICH Q2(R1) Validation of Analytical Procedures: Text and Methodology
2. EMEA/CHMP/EWP/192217/2009 Guideline on bioanalytical method validation
Calibration
The human periostin immunoassay is calibrated against recombinant human periostin peptide
Detection Limit & Sensitivity
To determine the sensitivity of the human periostin ELISA, experiments measuring the Lower Limit of Detection (LOD) and the lower limit of quantification (LLOQ) were conducted.
The LOD, also called the detection limit, is the lowest point at which a signal can be distinguished above the background signal, i.e. the signal that is measured in the absence of periostin, with a confidence level of 99%. It is defined as the mean back calculated concentration a periostin-free sample (three independent measurements) plus three times the standard deviation of the measurements.
The LLOQ, or sensitivity of an assay, is the lowest concentration at which an analyte can be accurately quantified. The criteria for accurate quantification at the LLOQ are an analyte recovery between 75 and 125% and a coefficient of variation (CV) of less than 25%. The lowest concentration of periostin, which meets both criteria, is reported as the LLOQ.
The following values were determined for the human periostin ELISA:
|
LOD |
20 pmol/l |
|
LLOQ |
62.5 pmol/l |
Precision
The precision of an ELISA is defined as its ability to measure the same concentration consistently within the same experiments carried out by one operator (within-run precision or repeatability) and across several experiments using the same samples but conducted by several operators at different locations using different ELISA lots (in-between-run precision or reproducibility).
Within-Run Precision
Within-run precision was tested by measuring the same samples 5 times within one human periostin ELISA lot. The experiment was conducted by one operator.
|
Within-Run Precision |
||
|
Sample |
#1 |
#2 |
|
n |
5 |
5 |
|
Mean [nmol/l] |
249 |
2008 |
|
Standard deviation [nmol/l] |
7.3 |
52 |
|
% CV |
3 |
3 |
In-Between-Run Precision
In-between-run precision was assessed by measuring the same samples 12 times within multiple human periostin ELISA lots. The measurements were carried out by multiple operators.
|
Within-Run Precision |
||
|
Sample |
#1 |
#2 |
|
n |
5 |
5 |
|
Mean [nmol/l] |
250 |
1996 |
|
Standard deviation [nmol/l] |
11.2 |
111.5 |
|
% CV |
4 |
6 |
Accuracy
The accuracy of an ELISA is defined as the precision with which it can recover samples of known concentrations.
The recovery of the periostin ELISA was measured by adding recombinant periostin to human samples containing a known concentration endogenous periostin. The % recovery of the spiked concentration was calculated as the percentage of measured compared over the expected value.
This table shows the summary of the recovery experiments in the human periostin ELISA in different sample matrices:
|
% Recovery |
|||||
|
Sample Matrix |
n |
+500 pmol/l |
+2000 pmol/l |
||
|
Mean |
Range |
Mean |
Range |
||
|
Serum |
7 |
106 |
88 - 127 |
95 |
91 - 105 |
|
EDTA plasma |
8 |
98 |
71 - 124 |
83 |
71 - 91 |
|
Heparin plasma |
7 |
92 |
84 - 104 |
85 |
75 - 96 |
|
Citrate plasma |
8 |
102 |
82 - 108 |
91 |
81 - 109 |
|
|
Periostin [pmol/l] |
% Recovery |
||||
|
Sample Matrix |
ID |
Reference |
+500 pmol/l |
+2000 pmol/l |
+500 pmol/l |
+2000 pmol/l |
|
Serum |
s1 |
688 |
1044 |
2170 |
88 |
91 |
|
Serum |
s2 |
927 |
1251 |
2270 |
88 |
90 |
|
Serum |
s3 |
892 |
1417 |
2266 |
127 |
91 |
|
Serum |
s4 |
881 |
1257 |
2537 |
97 |
105 |
|
Serum |
s5 |
1138 |
1623 |
2500 |
126 |
97 |
|
Serum |
s6 |
1009 |
1471 |
2445 |
118 |
97 |
|
Serum |
s7 |
1107 |
1470 |
2437 |
100 |
94 |
|
|
|
|
|
Mean |
106 |
95 |
|
|
|
|
|
Min |
88 |
91 |
|
|
|
|
|
Max |
127 |
105 |
Data showing recovery of periostin in human EDTA plasma samples:
|
Periostin [pmol/l] |
% Recovery |
|||||
|
Sample Matrix |
ID |
Reference |
+500 pmol/l |
+2000 pmol/l |
+500 pmol/l |
+2000 pmol/l |
|
EDTA plasma |
e1 |
907 |
1272 |
2266 |
96 |
91 |
|
EDTA plasma |
e2 |
542 |
1083 |
1980 |
122 |
85 |
|
EDTA plasma |
e3 |
774 |
1272 |
2021 |
119 |
82 |
|
EDTA plasma |
e4 |
784 |
1304 |
2187 |
124 |
90 |
|
EDTA plasma |
e5 |
789 |
1045 |
2321 |
71 |
96 |
|
EDTA plasma |
e6 |
1145 |
1356 |
2116 |
71 |
77 |
|
EDTA plasma |
e7 |
968 |
1256 |
1917 |
82 |
72 |
|
EDTA plasma |
e8 |
1008 |
1390 |
1921 |
102 |
71 |
|
|
|
|
|
Min |
71 |
71 |
|
|
|
|
|
Max |
124 |
91 |
Data showing recovery of periostin in human heparin plasma samples:
|
Periostin [pmol/l] |
% Recovery |
|||||
|
Sample Matrix |
ID |
Reference |
+500 pmol/l |
+2000 pmol/l |
+500 pmol/l |
+2000 pmol/l |
|
Heparin plasma |
h1 |
815 |
1201 |
2090 |
97 |
84 |
|
Heparin plasma |
h2 |
581 |
976 |
1878 |
94 |
79 |
|
Heparin plasma |
h3 |
765 |
1099 |
2090 |
86 |
85 |
|
Heparin plasma |
h4 |
808 |
1150 |
1908 |
89 |
75 |
|
Heparin plasma |
h5 |
831 |
1147 |
2345 |
84 |
96 |
|
Heparin plasma |
h6 |
780 |
1147 |
2067 |
93 |
84 |
|
Heparin plasma |
h7 |
998 |
1391 |
2304 |
104 |
90 |
|
|
|
|
|
Mean |
92 |
85 |
|
|
|
|
|
Min |
84 |
75 |
|
|
|
|
|
Max |
104 |
96 |
Data showing recovery of periostin in human citrate plasma samples:
|
Periostin [pmol/l] |
% Recovery |
|||||
|
Sample Matrix |
ID |
Reference |
+500 pmol/l |
+2000 pmol/l |
+500 pmol/l |
+2000 pmol/l |
|
Citrate plasma |
c1 |
798 |
1181 |
2036 |
97 |
82 |
|
Citrate plasma |
c2 |
519 |
956 |
1852 |
100 |
80 |
|
Citrate plasma |
c3 |
671 |
1074 |
2009 |
97 |
84 |
|
Citrate plasma |
c4 |
707 |
1136 |
1978 |
103 |
81 |
|
Citrate plasma |
c5 |
702 |
1113 |
2535 |
100 |
109 |
|
Citrate plasma |
c6 |
904 |
1332 |
2569 |
108 |
106 |
|
Citrate plasma |
c7 |
914 |
1209 |
2278 |
82 |
91 |
|
Citrate plasma |
c8 |
829 |
1369 |
2256 |
129 |
92 |
|
|
|
|
|
Mean |
102 |
91 |
|
|
|
|
|
Min |
82 |
81 |
|
|
|
|
|
Max |
129 |
109 |
Tests of dilution linearity and parallelism ensure that both endogenous and recombinant samples containing periostin behave in a dose dependent manner and are not affected by matrix effects. Dilution linearity assesses the accuracy of measurements in diluted human samples spiked with known concentrations of recombinant analyte. By contrast, parallelism refers to dilution linearity in human samples and provides evidence that the endogenous analyte behaves the same way as the recombinant one. For dilution linearity and parallelism are assessed for each sample type and are considered acceptable if the results are within 20% of the expected concentration.
Dilution linearity was assessed by serially diluting human samples spiked with 10 000 pmol/l recombinant human periostin.
The table below shows the mean recovery and range of serially diluted recombinant periostin in serum and citrate plasma. The reference value indicates the sample concentration before the spike.
|
Periostin [pmol/l] |
% Recovery |
|||||
|
Sample Matrix |
ID |
Reference |
1+4 |
1+9 |
1+4 |
1+9 |
|
Serum |
s1 |
1001 |
2342 |
1161 |
106 |
106 |
|
Serum |
s2 |
348 |
1765 |
956 |
85 |
92 |
|
Citrate Plasma |
c1 |
665 |
1774 |
776 |
83 |
73 |
|
Citrate Plasma |
c2 |
710 |
1918 |
858 |
90 |
80 |
Parallelism was assessed by serially diluting human samples containing endogenous periostin with assay buffer.
The table below shows the mean recovery and range of serially diluted endogenous periostin in several human sample matrices:
| % Recovery of endogenous periostin in diluted samples | |||||
|
1+1 |
1+3 |
||||
|
Sample Matrix |
ID |
Mean |
Range |
Mean |
Range |
|
Serum |
12 |
101 |
90 - 113 |
105 |
69 - 134 |
|
EDTA plasma |
4 |
99 |
97 - 101 |
115 |
110 - 117 |
|
Heparin plasma |
4 |
96 |
92 - 97 |
126 |
104 - 177 |
|
Citrate plasma |
4 |
95 |
92 - 101 |
122 |
117 - 124 |
|
Periostin [pmol/l] |
% Recovery |
|||||
|
Sample Matrix |
ID |
Reference |
1+1 |
1+3 |
1+1 |
1+3 |
|
Serum |
s1 |
647 |
308 |
121 |
95 |
93 |
|
Serum |
s2 |
1280 |
725 |
302 |
113 |
118 |
|
Serum |
s3 |
372 |
186 |
53 |
100 |
72 |
|
Serum |
s4 |
953 |
488 |
199 |
102 |
105 |
|
Serum |
s5 |
1440 |
713 |
316 |
99 |
110 |
|
Serum |
s6 |
1466 |
754 |
307 |
103 |
105 |
|
Serum |
s7 |
603 |
284 |
83 |
94 |
69 |
|
Serum |
s8 |
837 |
375 |
154 |
90 |
92 |
|
Serum |
s9 |
1608 |
846 |
511 |
105 |
127 |
|
Serum |
s10 |
1612 |
860 |
540 |
107 |
134 |
|
Serum |
s11 |
1155 |
618 |
337 |
107 |
117 |
|
Serum |
s12 |
1359 |
691 |
395 |
102 |
116 |
|
|
|
|
|
Mean |
101 |
105 |
|
|
|
|
|
Min |
90 |
69 |
|
|
|
|
|
Max |
113 |
134 |
Data showing dilution linearity of endogenous periostin in human EDTA plasma samples:
|
Periostin [pmol/l] |
% Recovery |
|||||
|
Sample Matrix |
ID |
Reference |
1+1 |
1+3 |
1+1 |
1+3 |
|
EDTA plasma |
e1 |
1468 |
714 |
431 |
97 |
117 |
|
EDTA plasma |
e2 |
1328 |
669 |
377 |
101 |
114 |
|
EDTA plasma |
e3 |
1222 |
614 |
359 |
100 |
117 |
|
EDTA plasma |
e4 |
1677 |
826 |
460 |
98 |
110 |
|
|
|
|
|
Mean |
99 |
115 |
|
|
|
|
|
Min |
97 |
110 |
|
|
|
|
|
Max |
101 |
117 |
Data showing dilution linearity of endogenous periostin in human heparin plasma samples:
|
Periostin [pmol/l] |
% Recovery |
|||||
|
Sample Matrix |
ID |
Reference |
1+1 |
1+3 |
1+1 |
1+3 |
|
Heparin plasma |
h1 |
1031 |
500 |
268 |
97 |
104 |
|
Heparin plasma |
h2 |
1230 |
597 |
544 |
97 |
177 |
|
Heparin plasma |
h3 |
1251 |
577 |
342 |
92 |
109 |
|
Heparin plasma |
h4 |
1417 |
682 |
407 |
96 |
115 |
|
|
|
|
|
96 |
126 |
Mean |
|
|
|
|
|
92 |
104 |
Min |
|
|
|
|
|
97 |
177 |
Max |
Data showing dilution linearity of endogenous periostin in human heparin plasma samples:
|
Periostin [pmol/l] |
% Recovery |
|||||
|
Sample Matrix |
ID |
Reference |
1+1 |
1+3 |
1+1 |
1+3 |
|
Citrate plasma |
c1 |
999 |
461 |
309 |
92 |
124 |
|
Citrate plasma |
c2 |
993 |
504 |
305 |
101 |
123 |
|
Citrate plasma |
c3 |
1079 |
507 |
328 |
94 |
122 |
|
Citrate plasma |
c4 |
1163 |
534 |
340 |
92 |
117 |
|
|
|
|
|
Mean |
95 |
122 |
|
|
|
|
|
Min |
92 |
117 |
|
|
|
|
|
Max |
101 |
124 |
Recommendations for sample dilution
High measuring samples outside of the calibration range of the curve should be diluted in assay buffer (ASYBUF, provided with the kit).
Specificity
The specificity of an ELISA is defined as its ability to exclusively recognize the analyte of interest.
The specificity of the human periostin ELISA was shown by characterizing both the capture and the detection antibodies through epitope mapping and affinity measurements. In addition, the specificity of the ELISA was established through competition experiments, which measure the ability of the antibodies to exclusively bind periostin.
This assay recognizes recombinant and endogenous (natural) human periostin.
ANTIBODY BINDING SITES
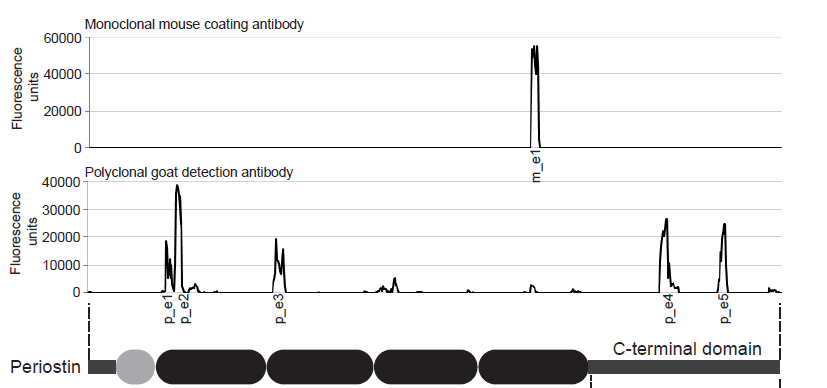
The detection antibody used in the human Periostin ELISA kit binds to a linear epitope close to the N-terminus in the FAS4 domain (AA541-549) of Periostin and the polyclonal coating antibody recognizes multiple linear epitopes distributed over the Periostin molecule.
A detailed description of the localization of epitops recognized by the antibodies used in this Periostin ELISA has been published (Gadermaier E et al., Characterization of a sandwich ELISA for the quantification of all human periostin isoforms. J Clin Lab Anal. 2018; 32(2). doi: 10.1002/jcla.22252.)
Epitop Mapping of Utilized Antibodies
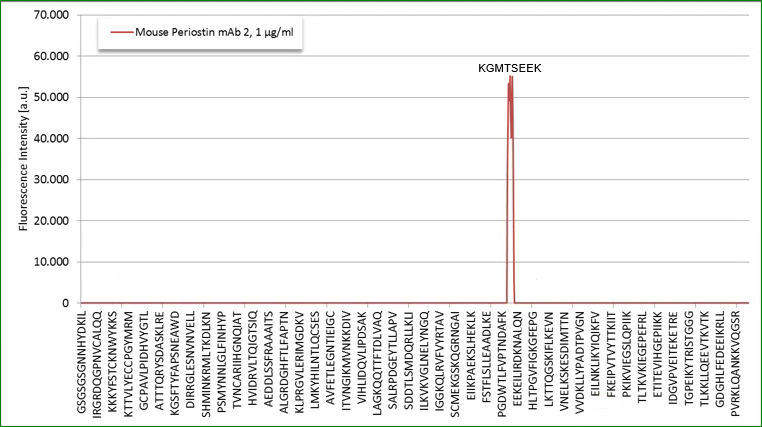
Epitop mapping has been performed by Pepperprint.
Coating antibody:
The coating antibody which is a monoclonal mouse antibody binds to m_e1: KGMTSEER (AA544-551) of Q62009-1 (Isoform 1).
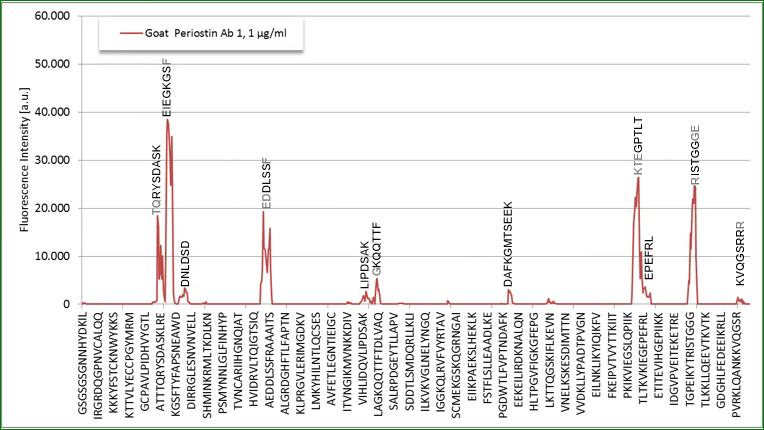
Detection antibody:
The detection antibody which is a polyclonal goat antibody binds to the following AA sequences of Q62009-1 (Isoform 1):
|
p_e1: TQHYSDVSK (AA115-123) |
|
p_e2: EIEGKGSY (AA127-134) |
|
p_e3: EDDLSSF (AA246-252) |
|
p_e4: PAMT (AA672-676) |
|
p_e5: RISTGGGE (AA738-746) |
Summary:
ISOFORMS
This assay is optimized to detect all known splicing forms of human periostin.
Cross-Reactivity
Due to the high sequence homology between human periostin and periostin of other species, the antibodies utilized in the assay may cross react with mouse, rat, cynomolgous monkey, dog and cat-periostin.
Sample Stability
Sample preparation
We recommend separating plasma or serum by centrifugation as soon as possible, e.g. 20 min at 2,000 x g, preferably at 4°C (2-8°C). Samples can be stored at 4°C (2-8°C) overnight. For long term storage, aliquot the acquired plasma or serum samples and store at -25°C or lower.
Freeze-thaw stability
The stability of endogenous periostin was tested by comparing periostin measurements in samples that had undergone four freeze-thaw cycles. The mean recovery of sample concentration after four freeze-thaw cycles is 96%.
| Mean % Recovery | |||||
|
Sample types |
n |
Reference |
2 x F/T |
3 x F/T |
4 x F/T |
|
Serum |
4 |
100 |
100 |
103 |
98 |
|
EDTA plasma |
4 |
100 |
91 |
93 |
90 |
|
Citrate plasma |
4 |
100 |
93 |
91 |
97 |
|
Heparin plasma |
4 |
100 |
96 |
96 |
99 |
|
|
|
|
|
Mean |
96 |
Samples can be subjected to 4 freeze-thaw cycles.
Sample Values
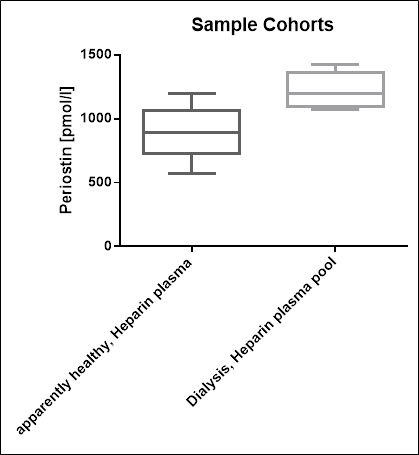
Serum and Plasma Periostin Values in Apparently Healthy Donors
To provide expected values for circulating periostin, a panel of samples from apparently healthy donors was tested.
A summary of the results is shown below:
|
Periostin [pmol/l] |
|||
|
Sample types |
n |
Median |
Range |
|
Serum |
24 |
864 |
397 – 1466 |
|
EDTA plasma |
20 |
817 |
532 – 1109 |
|
Citrate plasma |
20 |
891 |
569 – 1194 |
|
Heparin plasma |
24 |
885 |
321 - 1407 |
We recommended establishing the normal range for each laboratory.
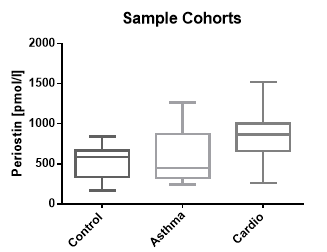
Periostin Values in Asthma and Cardiovascular Disease panels
In addition to samples from apparently healthy donors, a panel of samples from asthma and cardiovascular disease cohorts were tested.
The results of the experiments are shown below.
|
Periostin [pmol/l] |
|||
|
Sample types |
n |
Median |
Range |
|
Control |
18 |
586 |
165 – 840 |
|
Asthma Cohort* |
10 |
451 |
241 - 1266 |
|
Cardio Cohort* |
10 |
867 |
260 - 1521 |
*commercially available cohort (Seralab)
Matrix Comparison
Correlation of Serum and Plasma Samples from Apparently Healthy Individuals
14 samples of apparently healthy individuals were prepared as serum and plasma pairs each deriving from one donor. Samples were assayed, and the concentrations of the samples were compared.
|
Periostin [pmol/l] |
|
||||
|
Donor Sample ID |
Serum |
Citrate plasma |
EDTA plasma |
Heparin plasma |
CV (%) |
|
#1 |
1297 |
1081 |
1243 |
1195 |
7 |
|
#2 |
1303 |
1087 |
1267 |
1345 |
8 |
|
#3 |
1710 |
1337 |
1514 |
1512 |
9 |
|
#4 |
1946 |
1775 |
1899 |
2251 |
9 |
|
#5 |
1539 |
1273 |
1392 |
1108 |
12 |
|
#6 |
2279 |
1906 |
2265 |
2316 |
8 |
|
#7 |
1901 |
1636 |
1825 |
1712 |
6 |
|
#8 |
1879 |
1678 |
1822 |
2028 |
7 |
|
#9 |
2295 |
1890 |
2026 |
2102 |
7 |
|
#10 |
1572 |
1425 |
1520 |
1760 |
8 |
|
#11 |
801 |
669 |
749 |
788 |
7 |
|
#12 |
1013 |
883 |
1039 |
806 |
10 |
|
#13 |
915 |
796 |
965 |
881 |
7 |
|
#14 |
941 |
794 |
992 |
894 |
8 |
|
|
|
|
|
Mean |
8 |
-
Characterization of a sandwich ELISA for the quantification of all human periostin isoforms.
Gadermaier, E., Tesarz, M., Suciu, A.A.-M., Wallwitz, J., Berg, G., Himmler, G., 2018. J. Clin. Lab. Anal. 32.
PMID: 28493527
-
Gossiel, F., Scott, J.R., Paggiosi, M.A., Naylor, K.E., McCloskey, E.V., Peel, N.F.A., Walsh, J.S., Eastell, R., 2018. The Journal of Clinical Endocrinology & Metabolism 103, 1302–1309.
-
Mantovani, A., Sani, E., Fassio, A., Colecchia, A., Viapiana, O., Gatti, D., Idolazzi, L., Rossini, M., Salvagno, G., Lippi, G., Zoppini, G., Byrne, C.D., Bonora, E., Targher, G., 2018. Diabetes Metab.
PMID: 30315891
-
The Utility of Biomarkers in Osteoporosis Management.
Garnero, P., 2017. Mol Diagn Ther 21, 401–418.
-
Walsh, J.S., Gossiel, F., Scott, J.R., Paggiosi, M.A., Eastell, R., 2017. Bone 99, 8–13.
PMID: 28323143
-
A novel highly specific ELISA allows the measurement of human periostin in plasma and serum.
Sokolikova, B., Tesarz, M., Himmler, G., 2016. Presented at the 43rd Annual European Calcified Tissue Society Congress, BioScientifica.
-
With image
Image test
-
Test Title
Third review
-
Second review
My Review
-
First Title
First review
 Download biomedica product list 2018
Download biomedica product list 2018

 PMID: 28493527
PMID: 28493527





