FREE soluble RANKL HS ELISA
-
Method
Sandwich ELISA, HRP/TMB, 12×8-well detachable strips
-
Sample type
Serum, heparin plasma
-
Sample volume
150 µl / well
-
Assay time
2 h / overnight / 1 h / 30 min
-
Sensitivity
0.01 pmol/l (= 0.2 pg/ml)
-
Standard range
0 – 2 pmol/l (= 0 – 40 pg/ml)
-
Conversion factor
1 pg/ml = 0.05 pmol/l (MW: 20 kDa; monomer) or 1 pmol= 20 pg/ml
-
Specificity
Endogenous and recombinant free soluble RANKL
-
Precision
In-between-run (n=12): ≤ 3 % CV
Within-run (n=5): ≤ 5 % CV
-
Cross-reactivity
The sequence homology to various primates is >95%. It is likely that the assay can be used for these species.
-
Use
CE marked – for IVD use in the EU
-
Validation Data
See validation data tab for: precision, accuracy, dilution linearity, values for healthy donors, etc
Product Overview
The FREE soluble RANKL HS immunoassay is an overnight, 96-well sandwich ELISA for the quantitative determination of free soluble RANKL in serum and heparin plasma.
Principle of the Assay
This kit is a sandwich enzyme immunoassay for the quantitative determination of free soluble RANKL in human serum and heparin plasma samples.
The figure below explains the principle of the FREE soluble RANKL HS ELISA:
In a first step, wells which are pre-coated with recombinant Osteoprotegerin (OPG), RANKL’s interaction partner, are prewashed to ensure optimal sensitivity. Thereafter, assay buffer is pipetted into the wells of the microtiter strips followed by the addition of standard/control/sample. Any soluble RANKL present in the standard/control/sample binds to the pre-coated OPG in the well. After incubation, a washing step is applied where all non-specific unbound material is removed. In a next step, biotinylated detection antibody (polyclonal goat anti-sRANKL) is pipetted into the wells and reacts with the sRANKL present in the sample, forming a sandwich. Next, all unbound antibody is removed during another washing cycle. In the following step, the conjugate (streptavidin-polyHRP) is added and reacts with the detection antibody. After a final washing step, the substrate (TMB, tetramethylbenzidine) is pipetted into the wells. The enzyme-catalyzed color change of the substrate is directly proportional to the amount of soluble RANKL present in the sample. This color change is detectable with a standard microtiter plate reader. A dose response curve of the absorbance (optical density, OD at 450 nm) versus standard concentration is generated, using the values obtained from the standards. The concentration of soluble RANKL in the sample is determined directly from the dose response curve.
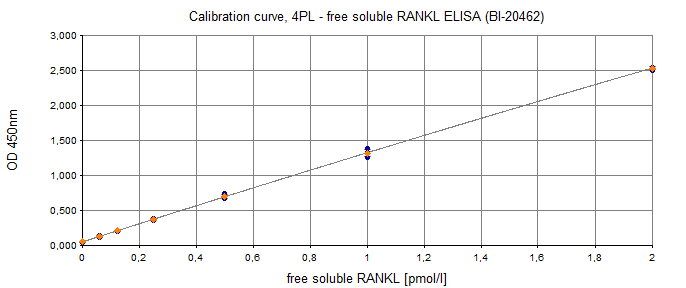
Typical Standard Curve
The figure below shows a typical standard curve for the free soluble RANKL ELISA. The immunoassay is calibrated against recombinant RANKL peptide:
ELISA Kit Components
|
Contents |
Description |
Quantity |
|
PLATE |
Human recombinant OPG pre-coated microtiter strips in strip holder, packed in aluminum bag with desiccant |
12 x 8 tests |
|
WASHBUF |
Wash buffer concentrate 20x, natural cap |
1 x 50 ml |
|
STD |
Standards (0; 0.0625; 0.125; 0.25; 0.5; 1; 2 pmol/l), lyophilized, white caps |
7 vials |
|
CTRL |
Control A+B, recombinant human RANKL in human serum, yellow caps, lyophilized, exact concentration see label |
2 vials |
|
ASYBUF |
Assay buffer, red cap, ready to use |
1 x 7 ml |
|
AB |
Goat polyclonal biotinylated anti-sRANKL antibody, green cap, ready to use |
1 x 22 ml |
|
CONJ |
Conjugate (streptavidin-polyHRP), amber cap, ready to use |
1 x 22 ml |
|
SUB |
Substrate (TMB solution), amber bottle, blue cap, ready to use |
1 x 22 ml |
|
STOP |
Stop solution, white cap, ready to use |
1 x 7 ml |
Storage instructions: All reagents of the free soluble RANKL ELISA kit are stable at 4°C until the expiry date stated on the label of each reagent.
Sample Collection & Storage
Serum and heparin plasma are suitable for use in this assay. Do not change sample type during studies. We recommend duplicate measurements for all samples, standards and controls. The sample collection and storage conditions listed are intended as general guidelines.
Serum & Plasma
Collect venous blood samples in standardized serum separator tubes (SST) or standardized blood collection tubes using EDTA, heparin or citrate as an anticoagulant. For serum samples, allow samples to clot for 30 minutes at room temperature. Perform separation by centrifugation according to the tube manufacturer’s instructions for use. Assay the acquired samples immediately or aliquot and store at -25°C or lower. Lipemic or haemolyzed samples may give erroneous results. Samples should undergo three freeze-thaw cycles only.
Reagent Preparation
Wash Buffer
|
1. |
Bring the WASHBUF concentrate to room temperature. Crystals in the buffer concentrate will dissolve at room temperature. |
|
2. |
Dilute the WASHBUF concentrate 1:20, e.g. 50 ml WASHBUF + 950 ml distilled or deionized water. Only use diluted WASHBUF when performing the assay. |
The diluted WASHBUF is stable up to one month at 4°C (2-8°C).
Standards & Controls
|
1. |
Pipette 700 µl of distilled or deionized water into each standard (STD) and control (CTRL) vial. The exact concentration is printed on the label of each vial. |
|
2. |
Leave at room temperature (18-26°C) for 15 min. Vortex gently. |
Reconstituted STDs and CTRLs are stable at -25°C or lower until expiry date stated on the label. STDs and CTRLs are stable for three freeze-thaw cycles.
Sample Preparation
Bring samples to room temperature and mix samples gently to ensure the samples are homogenous. We recommend duplicate measurements for all samples.
Samples for which the OD value exceeds the highest point of the standard range can be diluted with a serum sample with a low OD value.
Assay Protocol
Read the entire protocol before beginning the assay.
|
1. |
Bring reagents and samples to room temperature (18-26°C). |
|
2. |
Mark position for STD/CTRL/SAMPLE (standard/control/sample) on the protocol sheet. |
|
3. |
Take microtiter strips out of the aluminum bag. Store unused strips with desiccant at 4°C (2-8°C) in the aluminum bag. Strips are stable until expiry date stated on the label. |
|
4. |
Prewash wells with 300 µl diluted WASHBUF (wash buffer) five times. Remove remaining WASHBUF by strongly tapping plate against paper towel after the last wash. |
|
5. |
Pipette 50 µl ASYBUF (assay buffer, natural cap) into each well. |
|
6. |
Add 150 µl STD/CTRL/SAMPLE in duplicates into the respective wells. |
|
7. |
Cover the plate tightly and incubate for 2 hours at room temperature (18-26°C). |
|
8. |
Aspirate and wash wells 5x with 300 µl diluted WASHBUF. After the final wash, remove the remaining WASHBUF by strongly tapping plate against a paper towel. |
|
9. |
Add 200 µl AB (biotinylated anti-sRANKL antibody, green cap) into each well. Swirl gently. |
|
10. |
Cover tightly and incubate at 4°C (2-8°C) overnight (18-24 hours). |
|
11. |
Aspirate and wash wells 5x with 300 µl diluted WASHBUF. After the final wash, remove the remaining WASHBUF by strongly tapping plate against a paper towel. |
|
12. |
Add 200 µl CONJ (conjugate, amber cap) into each well, swirl gently. |
|
13. |
Cover tightly and incubate for 1 hour at room temperature in the dark. |
|
14. |
Aspirate and wash wells 5x with 300 µl diluted WASHBUF. After the final wash, remove remaining WASHBUF by strongly tapping plate against a paper towel. |
|
15. |
Add 200 µl SUB (substrate, blue cap) into each well. |
|
16. |
Incubate for 30 min at room temperature in the dark. |
|
17. |
Add 50 µl STOP (stop solution, white cap) into each well. |
|
18. |
Measure absorbance immediately at 450 nm with reference 630 nm, if available. |
Calculation of Results
Read the optical density (OD) of all wells on a plate reader using 450 nm wavelength (reference wavelength 630 nm). Construct a standard curve from the absorbance read-outs of the standards using commercially available software capable of generating a four-parameter logistic (4-PL) fit. Alternatively, plot the standards’ concentration on the x-axis against the mean absorbance for each standard on the y-axis and draw a best fit curve through the points on the graph. Curve fitting algorithms other than 4-PL have not been validated and will need to be evaluated by the user.
Obtain sample concentrations from the standard curve. If required, pmol/l can be converted to pg/ml by applying a conversion factor (sRANKL: 1 pg/ml = 0.05 pmol/l (MW: 20 kDa; monomer) or 1 pmol= 20 pg/ml). Respective dilution factors have to be considered when calculating the final concentration of the sample.
The quality control protocol supplied with the kit shows the results of the final release QC for each kit lot. Data for optical density obtained by customers may differ due to various influences including the normal decrease of signal intensity throughout shelf life. However, this does not affect validity of results as long as an OD of 1.50 or more is obtained for the standard with the highest concentration and the values of the CTRLs are within the target range (see labels).
INFORMATION ON THE ANALYTE
RANKL Protein
RANKL, the receptor activator of nuclear factor kappa B ligand, a member of the tumor necrosis factor (TNF) family (http://www.uniprot.org/uniprot/O14788), is the main stimulatory factor for the formation of mature osteoclasts and is essential for their survival. RANKL activates its specific receptor RANK, located on osteoclasts and dendritic cells. The effects are counteracted by Osteoprotegerin (OPG) which acts as an endogenous soluble receptor antagonist (see: OPG ELISA, cat.no. BI-20403).
The major source of RANKL are osteocytes, former osteoblasts that become embedded within the mineralized bone matrix. RANKL is a ~35 kD type II transmembrane-type protein and is cleaved to release a soluble biologically active product that forms a homotrimer.
|
Molecular Weight |
20 kDa (soluble form) |
|
Cellular localisation |
Intracellular, Membrane, Secreted |
|
Post-translational modifications |
Cleavage, glycosylation |
|
Sequence similarities |
Tumor necrosis factor (TNF) cytokine family |
|
Alternative Names |
TNF Superfamily Member, Tumor Necrosis Factor (Ligand) Superfamily, Member TNF-Related Activation-Induced Cytokine, Osteoclast Differentiation Factor, Osteoprotegerin Ligand, TRANCE, RANKL, OPGL, ODF, Receptor Activator Of Nuclear Factor Kappa B Ligand, Tumor Necrosis Factor Ligand Superfamily Member, Tumor Necrosis Factor Superfamily Member, Tumor Necrosis Factor Ligand 6B, CD254 Antigen, HRANKL, TNLG6B, CD254, OPTB2, SOdf |
|
Entrez/NCBI ID |
|
|
Genecards |
TNFSF11 |
|
OMIM |
|
|
PDB |
3URF http://www.rcsb.org/structure/3URF; |
|
Pfam |
PF00229: http://pfam.xfam.org/family/PF00229 |
|
Protein Atlas |
TNFSF11 https://www.proteinatlas.org/ENSG00000120659-TNFSF11/tissue |
|
Uniport ID |
RANKL Function
RANKL and its specific receptor RANK are not only key regulators of bone remodeling but also play an essential role in immunobiology, e.g. lymph node formation, establishment of the thymic microenvironment, mammary gland development during pregnancy, bone metastasis in cancer and sex-hormone, progestin-driven breast cancer, thermoregulation, and finally in the development of type 2 diabetes mellitus (Danks and Takayanagi, 2013; Gonzalez-Suarez et al., 2010; Hanada et al., 2010, 2009; Kiechl et al., 2016; Nakashima et al., 2011; Nelson et al., 2012; Schramek et al., 2010).
-
Bone Disease
Postmenopausal and senile osteoporosis (Anagnostis et al., 2018; Hofbauer and Schoppet, 2004; Stuss et al., 2016; Tella and Gallagher, 2014)
Glucocorticoid induced osteoporosis (Compston, 2018; Komori, 2016)
Disease with locally increased resorption activity
Rheumatoid Arthritis (Chiu and Ritchlin, 2017; Kurz et al., 2015; Remuzgo-Martínez et al., 2016; Tanaka et al., 2014; Tanaka and Ohira, 2018)
HIV associated bone loss (Negredo et al., 2015; Pietschmann et al., 2016; Titanji, 2017)
Ankylosing spondylitis (Caparbo et al., 2018; Perpétuo et al., 2017)
-
Cancer
Malignant solid tumors (de Groot et al., 2018)
Breast cancer (Kiechl et al., 2016, 2016; Lüftner et al., 2018; Rachner et al., 2018; Rao et al., 2018; Sarink et al., 2017; Sigl et al., 2016)
Multiple myeloma (Raje et al., 2019; Terpos et al., 2017)
-
Endocrine disorders
Test
-
Inflammation
Test
-
Inflammatory diseases
Test
-
Lung
Test
-
Metabolic Disease
Type 1 diabetes mellitus (Chrysis et al., 2017; Grzelak et al., 2018; Starup-Linde et al., 2016)
Type 2 diabetes mellitus (Grigoropoulou et al., 2011; Harper et al., 2016; Jansen et al., 2018; Mantovani et al., 2018)
-
Pulmonology
Test
-
Respiratory diseases
Test
Literature
Role of the RANK/RANKL Pathway in Multiple Myeloma.
Raje, N.S., Bhatta, S., Terpos, E., 2019. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 25, 12–20.
The role of sclerostin/dickkopf-1 and receptor activator of nuclear factor kB ligand/osteoprotegerin signalling pathways in the development of osteoporosis in patients with haemophilia A and B: A cross-sectional study.
Anagnostis, P., Vakalopoulou, S., Christoulas, D., Paschou, S.A., Papatheodorou, A., Garipidou, V., Kokkoris, P., Terpos, E., 2018. Haemophilia 24, 316–322.
Monocytes from male patients with ankylosing spondylitis display decreased osteoclastogenesis and decreased RANKL/OPG ratio.
Caparbo, V.F., Saad, C.G.S., Moraes, J.C., de Brum-Fernandes, A.J., Pereira, R.M.R., 2018. Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA.
Glucocorticoid-induced osteoporosis: an update.
Compston, J., 2018. Endocrine 61, 7–16.
The anti-tumor effect of RANKL inhibition in malignant solid tumors - A systematic review.
de Groot, A.F., Appelman-Dijkstra, N.M., van der Burg, S.H., Kroep, J.R., 2018. Cancer Treat. Rev. 62, 18–28.
Neuropeptide B and neuropeptide W as new serum predictors of nutritional status and of clinical outcomes in pediatric patients with type 1 diabetes mellitus treated with the use of pens or insulin pumps.
Grzelak, T., Wedrychowicz, A., Grupinska, J., Pelczynska, M., Sperling, M., Mikulska, A.A., Naughton, V., Czyzewska, K., 2018. Arch. Med. Sci. 14.
Bone mineral density and markers of bone turnover and inflammation in diabetes patients with or without a Charcot foot: An 8.5-year prospective case-control study.
Jansen, R.B., Christensen, T.M., Bülow, J., Rørdam, L., Holstein, P.E., Jørgensen, N.R., Svendsen, O.L., 2018. J. Diabetes Complications 32, 164–170.
Therapeutic approaches for protecting bone health in patients with breast cancer.
Lüftner, D., Niepel, D., Steger, G.G., 2018. Breast Edinb. Scotl. 37, 28–35.
Association between non-alcoholic fatty liver disease and bone turnover biomarkers in post-menopausal women with type 2 diabetes.
Mantovani, A., Sani, E., Fassio, A., Colecchia, A., Viapiana, O., Gatti, D., Idolazzi, L., Rossini, M., Salvagno, G., Lippi, G., Zoppini, G., Byrne, C.D., Bonora, E., Targher, G., 2018. Diabetes Metab.
Prognostic value of RANKL/OPG serum levels and disseminated tumor cells in non-metastatic breast cancer.
Rachner, T.D., Kasimir-Bauer, S., Göbel, A., Erdmann, K., Hoffmann, O., Browne, A.J., Wimberger, P., Rauner, M., Hofbauer, L.C., Kimmig, R., Bittner, A.-K., 2018. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res.
RANKL and RANK: From Mammalian Physiology to Cancer Treatment.
Rao, S., Cronin, S.J.F., Sigl, V., Penninger, J.M., 2018. Trends Cell Biol. 28, 213–223.
Mechanisms and therapeutic targets for bone damage in rheumatoid arthritis, in particular the RANK-RANKL system.
Tanaka, Y., Ohira, T., 2018. Curr. Opin. Pharmacol. 40, 110–119.
Denosumab: targeting the RANKL pathway to treat rheumatoid arthritis.
Chiu, Y.G., Ritchlin, C.T., 2017. Expert Opin. Biol. Ther. 17, 119–128.
Osteoprotegerin, RANKL, ADMA, and Fetuin-A serum levels in children with type I diabetes mellitus.
Chrysis, D., Efthymiadou, A., Mermigka, A., Kritikou, D., Spiliotis, B.E., 2017. Pediatr. Diabetes 18, 277–282.
Ankylosing Spondylitis Patients Have Impaired Osteoclast Gene Expression in Circulating Osteoclast Precursors.
Perpétuo, I.P., Caetano-Lopes, J., Vieira-Sousa, E., Campanilho-Marques, R., Ponte, C., Canhão, H., Ainola, M., Fonseca, J.E., 2017. Front. Med. 4.
Circulating RANKL and RANKL/OPG and Breast Cancer Risk by ER and PR Subtype: Results from the EPIC Cohort.
Sarink, D., Schock, H., Johnson, T., Overvad, K., Holm, M., Tjønneland, A., Boutron-Ruault, M.-C., His, M., Kvaskoff, M., Boeing, H., Lagiou, P., Papatesta, E.-M., Trichopoulou, A., Palli, D., Pala, V., Mattiello, A., Tumino, R., Sacerdote, C., Bueno-de-Mesquita, H.B.A., van Gils, C.H., Peeters, P.H., Weiderpass, E., Agudo, A., Sánchez, M.-J., Chirlaque, M.-D., Ardanaz, E., Amiano, P., Khaw, K.T., Travis, R., Dossus, L., Gunter, M., Rinaldi, S., Merritt, M., Riboli, E., Kaaks, R., Fortner, R.T., 2017. Cancer Prev. Res. Phila. Pa 10, 525–534.
Mechanisms of bone destruction in multiple myeloma.
Terpos, E., Christoulas, D., Gavriatopoulou, M., Dimopoulos, M.A., 2017. Eur. J. Cancer Care (Engl.) 26.
Beyond Antibodies: B Cells and the OPG/RANK-RANKL Pathway in Health, Non-HIV Disease and HIV-Induced Bone Loss.
Titanji, K., 2017. Front. Immunol. 8, 1851.
Vascular calcification in type-2 diabetes and cardiovascular disease: Integrative roles for OPG, RANKL and TRAIL.
Harper, E., Forde, H., Davenport, C., Rochfort, K.D., Smith, D., Cummins, P.M., 2016. Vascul. Pharmacol. 82, 30–40.
Aberrant regulation of RANKL/OPG in women at high risk of developing breast cancer.
Kiechl, S., Schramek, D., Widschwendter, M., Fourkala, E.-O., Zaikin, A., Jones, A., Jaeger, B., Rack, B., Janni, W., Scholz, C., Willeit, J., Weger, S., Mayr, A., Teschendorff, A., Rosenthal, A., Fraser, L., Philpott, S., Dubeau, L., Keshtgar, M., Roylance, R., Jacobs, I.J., Menon, U., Schett, G., Penninger, J.M., 2016. Oncotarget 8, 3811–3825.
Glucocorticoid Signaling and Bone Biology.
Komori, T., 2016. Horm. Metab. Res. Horm. Stoffwechselforschung Horm. Metab. 48, 755–763.
Immunology of Osteoporosis: A Mini-Review.
Pietschmann, P., Mechtcheriakova, D., Meshcheryakova, A., Föger-Samwald, U., Ellinger, I., 2016. Gerontology 62, 128–137.
Expression of osteoprotegerin and its ligands, RANKL and TRAIL, in rheumatoid arthritis.
Remuzgo-Martínez, S., Genre, F., López-Mejías, R., Ubilla, B., Mijares, V., Pina, T., Corrales, A., Blanco, R., Martín, J., Llorca, J., González-Gay, M.A., 2016. Sci. Rep. 6, 29713.
RANKL/RANK: from bone loss to the prevention of breast cancer.
Sigl, V., Jones, L.P., Penninger, J.M., 2016. Open Biol. 6.
Differences in biochemical bone markers by diabetes type and the impact of glucose.
Starup-Linde, J., Lykkeboe, S., Gregersen, S., Hauge, E.-M., Langdahl, B.L., Handberg, A., Vestergaard, P., 2016. Bone 83, 149–155.
Assessment of OPG, RANKL, bone turnover markers serum levels and BMD after treatment with strontium ranelate and ibandronate in patients with postmenopausal osteoporosis.
Stuss, M., Sewerynek, E., Król, I., Stępień-Kłos, W., Jędrzejczyk, S., 2016. Endokrynol. Pol. 67, 174–184.
Effects of Antitumor Necrosis Factor Therapy on Osteoprotegerin, Neopterin, and sRANKL Concentrations in Patients with Rheumatoid Arthritis.
Kurz, K., Herold, M., Russe, E., Klotz, W., Weiss, G., Fuchs, D., 2015. Dis. Markers, 276969.
Switching from tenofovir to abacavir in HIV-1-infected patients with low bone mineral density: changes in bone turnover markers and circulating sclerostin levels.
Negredo, E., Diez-Pérez, A., Bonjoch, A., Domingo, P., Pérez-Álvarez, N., Gutierrez, M., Mateo, G., Puig, J., Echeverría, P., Escrig, R., Clotet, B., 2015. J. Antimicrob. Chemother. 70, 2104–2107.
IL-6 in Inflammation, Immunity, and Disease.
Tanaka, T., Narazaki, M., Kishimoto, T., 2014. Cold Spring Harb. Perspect. Biol. 6, a016295–a016295
Prevention and treatment of postmenopausal osteoporosis.
Tella, S.H., Gallagher, J.C., 2014. J. Steroid Biochem. Mol. Biol. 142, 155–170.
Immunology and bone.
Danks, L., Takayanagi, H., 2013. J. Biochem. (Tokyo) 154, 29–39.
RANKL employs distinct binding modes to engage RANK and the osteoprotegerin decoy receptor.
Nelson, C.A., Warren, J.T., Wang, M.W.-H., Teitelbaum, S.L., Fremont, D.H., 2012. Struct. Lond. Engl. 1993 20, 1971–1982.
The role of the osteoprotegerin/RANKL/RANK system in diabetic vascular disease.
Grigoropoulou, P., Eleftheriadou, I., Zoupas, C., Tentolouris, N., 2011. Curr. Med. Chem. 18, 4813–4819
Evidence for osteocyte regulation of bone homeostasis through RANKL expression.
Nakashima, T., Hayashi, M., Fukunaga, T., Kurata, K., Oh-Hora, M., Feng, J.Q., Bonewald, L.F., Kodama, T., Wutz, A., Wagner, E.F., Penninger, J.M., Takayanagi, H., 2011. Nat. Med. 17, 1231–1234.
RANK ligand mediates progestin-induced mammary epithelial proliferation and carcinogenesis.
Gonzalez-Suarez, E., Jacob, A.P., Jones, J., Miller, R., Roudier-Meyer, M.P., Erwert, R., Pinkas, J., Branstetter, D., Dougall, W.C., 2010. Nature 468, 103–107.
Physiology and pathophysiology of the RANKL/RANK system.
Hanada, R., Hanada, T., Penninger, J.M., 2010. Biol. Chem. 391, 1365–1370.
Osteoclast differentiation factor RANKL controls development of progestin-driven mammary cancer.
Schramek, D., Leibbrandt, A., Sigl, V., Kenner, L., Pospisilik, J.A., Lee, H.J., Hanada, R., Joshi, P.A., Aliprantis, A., Glimcher, L., Pasparakis, M., Khokha, R., Ormandy, C.J., Widschwendter, M., Schett, G., Penninger, J.M., 2010. Nature 468, 98–102.
Central control of fever and female body temperature by RANKL/RANK.
Hanada, R., Leibbrandt, A., Hanada, T., Kitaoka, S., Furuyashiki, T., Fujihara, H., Trichereau, J., Paolino, M., Qadri, F., Plehm, R., Klaere, S., Komnenovic, V., Mimata, H., Yoshimatsu, H., Takahashi, N., von Haeseler, A., Bader, M., Kilic, S.S., Ueta, Y., Pifl, C., Narumiya, S., Penninger, J.M., 2009. Nature 462, 505–509.
Clinical implications of the osteoprotegerin/RANKL/RANK system for bone and vascular diseases.
Hofbauer, L.C., Schoppet, M., 2004. JAMA 292, 490–495.
All Biomedica ELISAs are validated according to international FDA/ICH/EMEA guidelines. For more information about our validation guidelines, please refer to our quality page and published validation guidelines and literature.
- CPMP/ICH/381/95: ICH Q2(R1) Validation of Analytical Procedures: Text and Methodology
- EMEA/CHMP/EWP/192217/2009 Guideline on bioanalytical method validation
- Bioanalytical Method Validation, Guidance for Industry, FDA, May 2018
Calibration
The free soluble RANKL immunoassay is calibrated against human recombinant RANKL protein.
Detection Limit & Sensitivity
To determine the sensitivity of the free soluble RANKL ELISA, experiments measuring the lower limit of detection (LOD) and the lower limit of quantification (LLOQ) were conducted.
The LOD, also called the detection limit, is the lowest point at which a signal can be distinguished above the background signal, i.e. the signal that is measured in the absence of RANKL, with a confidence level of 99%.
The LLOQ, or sensitivity of an assay, is the lowest concentration at which an analyte can be accurately quantified. The criteria for accurate quantification at the LLOQ are an analyte recovery between 75 and 125% and a coefficient of variation (CV) of less than 25%.
The following values were determined for the free soluble RANKL ELISA:
|
LOD |
0.01 pmol/l |
|
LLOQ |
0.008 pmol/l |
Independently, an external clinical research organization (CRO) determined the LOD as 0.009 pmol/l and the LLOQ at 0.0038 pmol/l.
The lower limit of detection (LOD) of free sRANKL is 0.009 pmol/l and determined by adding 3 SD to the mean of a standard zero study.
|
Measurement |
Soluble RANKL [pmol/l] |
|
1 |
0.003 |
|
2 |
0.008 |
|
3 |
0.001 |
|
4 |
0.002 |
|
5 |
0.003 |
|
6 |
0.002 |
|
7 |
0.001 |
|
8 |
0.001 |
|
9 |
0.002 |
|
10 |
0.002 |
|
11 |
0.003 |
|
12 |
0.003 |
|
13 |
0.003 |
|
14 |
0.002 |
|
15 |
0.002 |
|
16 |
0.003 |
|
17 |
0.002 |
|
Mean |
0.003 |
|
SD |
0.002 |
|
% CV |
62.7% |
|
LOD |
0.009 |
The lower limit of quantitation was determined by the Sample B and verified by QC1, Sample A and QC 1-2.
|
Measurement |
QC1 [pmol/l] |
Sample A [pmol/l] |
Sample B [pmol/l] |
QC 1-2 [pmol/l] |
|
1 |
0.046 |
0.044 |
0.035 |
0.020 |
|
2 |
0.045 |
0.054 |
0.038 |
0.027 |
|
3 |
0.046 |
0.050 |
0.038 |
0.014 |
|
4 |
0.045 |
0.048 |
0.034 |
0.016 |
|
5 |
0.048 |
0.050 |
0.034 |
0.017 |
|
6 |
0.046 |
0.047 |
0.043 |
0.021 |
|
7 |
0.039 |
0.047 |
0.033 |
0.026 |
|
8 |
0.046 |
0.046 |
0.035 |
0.021 |
|
9 |
0.046 |
0.048 |
0.040 |
0.015 |
|
10 |
0.045 |
0.052 |
0.033 |
0.015 |
|
11 |
0.046 |
0.047 |
0.040 |
0.017 |
|
12 |
0.045 |
0.050 |
0.042 |
0.020 |
|
13 |
0.046 |
0.047 |
0.042 |
0.028 |
|
14 |
0.045 |
0.046 |
0.040 |
0.028 |
|
15 |
0.046 |
- |
0.040 |
- |
|
Mean [pmol/l]: |
0.045 |
0.048 |
0.038 |
0.020 |
|
SD [pmol/l]: |
0.002 |
0.003 |
0.004 |
0.005 |
|
CV [%]: |
4.2% |
5.5% |
9.3% |
24.9% |
Precision
The precision of an ELISA is defined as its ability to measure the same concentration consistently within the same experiments carried out by one operator (within-run precision or repeatability) and across several experiments using the same samples but conducted by several operators using different ELISA lots (in-between-run precision or reproducibility).
Within-Run Precision (repeatability or intra-assay precision)
Within-run / intra-assay precision was assessed by measuring 2 samples of known concentrations 5 times within one free soluble RANKL ELISA kit lot by one operator.
|
Intra-assay (n=5) |
Sample 1 |
Sample 2 |
|
Mean [pmol/l] |
0.12 |
0.98 |
|
SD [pmol/l] |
0.006 |
0.009 |
|
CV (%) |
5 |
1 |
Independently, within-run / intra-assay precision was assessed by an independent CRO by measuring 5 samples 15 times.
|
Intra-assay (n=15) |
Sample 1 |
Sample 2 |
Sample 3 |
Sample 4 |
Sample 5 |
|
Mean [pmol/l] |
0.045 |
0.102 |
0.142 |
0.242 |
0.628 |
|
SD [pmol/l] |
0.002 |
0.007 |
0.006 |
0.006 |
0.008 |
|
CV (%) |
4.2 |
6.6 |
4.4 |
2.5 |
1.3 |
|
Measurement |
QC1 [pmol/l] |
QC2 [pmol/l] |
QC3 [pmol/l] |
QC4 [pmol/l] |
QC5 [pmol/l] |
|
1 |
0.046 |
0.098 |
0.149 |
0.245 |
0.620 |
|
2 |
0.045 |
0.098 |
0.146 |
0.242 |
0.609 |
|
3 |
0.046 |
0.103 |
0.137 |
0.240 |
0.621 |
|
4 |
0.045 |
0.092 |
0.142 |
0.246 |
0.632 |
|
5 |
0.048 |
0.102 |
0.146 |
0.233 |
0.636 |
|
6 |
0.046 |
0.103 |
0.135 |
0.242 |
0.629 |
|
7 |
0.039 |
0.099 |
0.135 |
0.244 |
0.642 |
|
8 |
0.046 |
0.105 |
0.141 |
0.254 |
0.625 |
|
9 |
0.046 |
0.104 |
0.138 |
0.233 |
0.627 |
|
10 |
0.045 |
0.095 |
0.141 |
0.241 |
0.627 |
|
11 |
0.046 |
0.102 |
0.150 |
0.242 |
0.638 |
|
12 |
0.045 |
0.119 |
0.132 |
0.233 |
0.624 |
|
13 |
0.046 |
- |
0.141 |
0.237 |
0.630 |
|
14 |
0.045 |
- |
0.144 |
0.250 |
0.627 |
|
15 |
0.046 |
- |
0.155 |
0.246 |
0.634 |
|
Mean [pmol/l] |
0.045 |
0.102 |
0.142 |
0.242 |
0.628 |
|
SD [pmol/l] |
0.002 |
0.007 |
0.006 |
0.006 |
0.008 |
|
CV % |
4.2 |
6.6 |
4.4 |
2.5 |
1.3 |
In-Between-Run Precision (inter-assay precision)
In-between-run /intra-assay precision was assessed by measuring 2 samples 12 times within free soluble RANKL ELISA kit lots by three different operators.
|
Inter-assay (n=2) |
Sample 1 |
Sample 2 |
|
Mean [pmol/l] |
0.12 |
1.00 |
|
SD [pmol/l] |
0.004 |
0.02 |
|
CV (%) |
3 |
2 |
Independently, in-between-run /intra-assay precision was assessed by an independent CRO by measuring 4 samples of known concentrations 7 times.
|
Intra-assay (n=7) |
Sample 1 |
Sample 2 |
Sample 3 |
Sample 4 |
|
Mean [pmol/l] |
0.114 |
0.161 |
0.241 |
0.550 |
|
SD [pmol/l] |
0.02 |
0.01 |
0.00 |
0.0 |
|
CV (%) |
16.2 |
6.3 |
1.8 |
2.1 |
|
Measurement |
QC1 [pmol/l] |
QC2 [pmol/l] |
QC3 [pmol/l] |
QC4 [pmol/l] |
|
1 |
0.093 |
0.145 |
0.239 |
0.545 |
|
2 |
- |
0.151 |
0.242 |
0.544 |
|
3 |
- |
0.160 |
0.249 |
0.573 |
|
4 |
0.101 |
0.164 |
0.239 |
0.545 |
|
5 |
0.113 |
0.165 |
0.245 |
0.538 |
|
6 |
0.139 |
0.176 |
0.237 |
0.557 |
|
7 |
0.126 |
0.163 |
0.238 |
0.550 |
|
Mean [pmol/l] |
0.114 |
0.161 |
0.241 |
0.550 |
|
SD [pmol/l] |
0.02 |
0.01 |
0.00 |
0.0 |
|
CV % |
16.2 |
6.3 |
1.8 |
2.1 |
Accuracy
The accuracy of an ELISA is defined as the precision with which it can recover samples of known concentrations.
The recovery of the human free soluble RANKL ELISA was measured by adding human recombinant soluble RANKL to human samples containing a known concentration endogenous RANKL. The % recovery of the spiked concentration was calculated as the percentage of measured compared over the expected value.
This table shows the summary of the recovery experiments in the free soluble RANKL ELISA in different sample matrices:
|
|
% Recovery |
||||
|
|
|
+ 0.25 pmol/l |
+ 1 pmol/l |
||
|
Sample Matrix |
n |
Mean |
Range |
Mean |
Range |
|
Serum |
8 |
91 |
70 – 104 |
84 |
67 – 100 |
|
Heparin plasma |
8 |
91 |
67 – 119 |
83 |
67 - 106 |
Please note: Recovery of recombinant RANKL is a complicated issue in serum/plasma samples as the samples contain a binding factor that influences the recovery. This observation is different from a sample spiked with endogenous soluble RANKL, as the sample has already reached its equilibrium with OPG (and other potential binding factors).
Data showing % recovery of recombinant RANKL in human serum samples:
|
RANKL c[pmol/l] |
% Recovery |
|||||
|
Sample Matrix |
ID |
Reference |
+0.25 pmol/l |
+1 pmol/l |
+0.25 pmol/l |
+1 pmol/l |
|
Serum |
s1 |
1.01 |
1.19 |
1.70 |
70 |
70 |
|
Serum |
s2 |
0.47 |
0.73 |
1.47 |
104 |
104 |
|
Serum |
s3 |
0.27 |
0.53 |
1.20 |
103 |
103 |
|
Serum |
s4 |
0.23 |
0.43 |
1.04 |
77 |
77 |
|
Serum |
s5 |
0.05 |
0.29 |
0.83 |
98 |
98 |
|
Serum |
s6 |
0.07 |
0.25 |
0.74 |
70 |
70 |
|
Serum |
s7 |
0.52 |
0.76 |
1.39 |
98 |
98 |
|
Serum |
s8 |
0.27 |
0.48 |
1.23 |
85 |
85 |
|
Mean |
91 |
84 |
||||
|
Min |
70 |
67 |
||||
|
Max |
104 |
100 |
||||
Data showing % recovery of recombinant RANKL in human heparin plasma samples:
|
RANKL c[pmol/l] |
% Recovery |
|||||
|
Sample Matrix |
ID |
Reference |
+0.25 pmol/l |
+1 pmol/l |
+0.25 pmol/l |
+1 pmol/l |
|
Heparin plasma |
h1 |
0.31 |
0.55 |
1.29 |
98 |
98 |
|
Heparin plasma |
h2 |
0.18 |
0.44 |
0.99 |
102 |
81 |
|
Heparin plasma |
h3 |
0.39 |
0.64 |
1.42 |
98 |
103 |
|
Heparin plasma |
h4 |
0.49 |
0.79 |
1.55 |
119 |
106 |
|
Heparin plasma |
h5 |
0.06 |
0.23 |
0.73 |
67 |
67 |
|
Heparin plasma |
h6 |
0.04 |
0.24 |
0.89 |
79 |
85 |
|
Heparin plasma |
h7 |
0.19 |
0.37 |
0.96 |
74 |
77 |
|
Heparin plasma |
h8 |
0.10 |
0.31 |
0.85 |
84 |
76 |
|
Mean |
91 |
86 |
||||
|
Min |
67 |
67 |
||||
|
Max |
119 |
106 |
||||
Accuracy was also assessed by an external CRO in serum and recovery was determined to be between 80 and 113%.
Experiment: Recovery was determined by mixing samples with different standards in a 75:25 ratio.
|
|
Sample value [pmol/l] |
Added values [pmol/l] |
Expected values [pmol/l] |
Assay values [pmol/l] |
Recovery [%] |
|
Sample C |
0.113 |
0.125 |
0.116 |
0.131 |
113 |
|
0.250 |
0.148 |
0.148 |
100 |
||
|
0.500 |
0.210 |
0.215 |
102 |
||
|
1.000 |
0.335 |
0.298 |
89 |
||
|
2.000 |
0.585 |
0.480 |
82 |
||
|
Sample D |
0.118 |
0.125 |
0.120 |
0.115 |
96 |
|
0.250 |
0.152 |
0.138 |
91 |
||
|
0.500 |
0.214 |
0.184 |
86 |
||
|
1.000 |
0.339 |
0.274 |
81 |
||
|
2.000 |
0.589 |
0.472 |
80 |
||
|
|
|
|
|
Mean R [%]: |
92 |
Parallelism
Tests of parallelism ensure that samples containing endogenous soluble RANKL behave in a dose dependent manner and are not affected by matrix effects. Parallelism refers to dilution linearity in clinical samples.
Parallelism was assessed by serially diluting samples containing endogenous soluble RANKL with serum-based standard matrix.
|
|
|
% Recovery of endogenous soluble RANKL in diluted samples |
|
|
|
|
1+1 |
1+3 |
|
Sample Matrix |
n |
Mean |
Mean |
|
Serum |
8 |
112 |
135 |
|
Heparin plasma |
8 |
121 |
- |
|
Serum |
8 |
112 |
81-113 |
|
Soluble RANKL [pmol/l] |
% Recovery |
|||||
|
Sample matrix |
ID |
Reference |
1+1 |
1+3 |
1+1 |
1+3 |
|
Serum |
s1 |
647 |
308 |
121 |
95 |
93 |
|
Serum |
s2 |
1280 |
725 |
302 |
113 |
118 |
|
Serum |
s3 |
372 |
186 |
53 |
100 |
72 |
|
Serum |
s4 |
953 |
488 |
199 |
102 |
105 |
|
Serum |
s5 |
1440 |
713 |
316 |
99 |
110 |
|
Serum |
s6 |
1466 |
754 |
307 |
103 |
105 |
|
Serum |
s7 |
603 |
284 |
83 |
94 |
69 |
|
Serum |
s8 |
837 |
375 |
154 |
90 |
92 |
|
Serum |
s9 |
1608 |
846 |
511 |
105 |
127 |
|
Serum |
s10 |
1612 |
860 |
540 |
107 |
134 |
|
Serum |
s11 |
1155 |
618 |
337 |
107 |
117 |
|
Serum |
s12 |
1359 |
691 |
395 |
102 |
116 |
|
Mean |
101 |
105 |
||||
|
Min |
90 |
69 |
||||
|
Max |
113 |
134 |
||||
Parallelism was independently validated in serum by an external CRO:
|
|
|
% Recovery of endogenous soluble RANKL in diluted samples |
|
|
1+1 |
|||
|
Sample Matrix |
n |
Mean |
SD |
|
Serum |
8 |
96 |
±12 |
Diluted in a very low pool serum, results not corrected by dilution factor (2).
|
Sample |
Dilution |
free sRANKL [pmol/l] |
Recovery [%] from neat |
|
Pool A |
neat |
0.229 |
- |
|
1/2 |
0.093 |
81 |
|
|
Pool B |
neat |
0.243 |
- |
|
1/2 |
0.110 |
91 |
|
|
Pool C |
neat |
0.258 |
- |
|
1/2 |
0.113 |
88 |
|
|
Pool D |
neat |
0.199 |
- |
|
1/2 |
0.098 |
98 |
|
|
Sample 1 |
neat |
0.300 |
- |
|
1/2 |
0.169 |
113 |
|
|
Sample 2 |
neat |
0.229 |
- |
|
1/2 |
0.120 |
105 |
|
|
|
|
Mean R [%] |
96 |
|
± |
12 |
Specificity
The specificity of an ELISA is defined as its ability to exclusively recognize the analyte of interest.
The specificity of the free soluble RANKL ELISA was established through competition experiments, which measure the ability of the antibodies to exclusively bind soluble RANKL.
Competition of Signal
Competition experiments were carried out by pre-incubating human samples with an excess of OPG. The concentration measured in this mixture was then compared to a reference value, which was obtained from the same sample but without the pre-incubation step. Mean competition was 100 % in both serum and heparin plasma.
Serum:
|
Soluble RANKL [pmol/l] |
|
|||
|
Sample matrix |
ID |
Reference |
Reference + 1µl OPG |
% Competition |
|
Serum |
s1 |
1.01 |
0.00 |
100 |
|
Serum |
s2 |
0.47 |
0.00 |
100 |
|
Serum |
s3 |
0.27 |
0.00 |
100 |
|
Serum |
s4 |
0.23 |
0.00 |
100 |
|
Serum |
s5 |
0.05 |
0.00 |
90 |
|
Serum |
s6 |
0.07 |
0.00 |
100 |
|
Serum |
s7 |
0.52 |
0.03 |
94 |
|
Serum |
s8 |
0.27 |
0.00 |
100 |
|
Serum |
s9 |
0.37 |
0.00 |
99 |
|
Serum |
s10 |
0.47 |
0.00 |
100 |
|
Serum |
s11 |
0.40 |
0.01 |
99 |
|
Serum |
s12 |
0.10 |
0.00 |
100 |
|
Serum |
s13 |
0.45 |
0.00 |
99 |
|
Mean |
100 |
|||
Heparin plasma:
|
Soluble RANKL [pmol/l] |
|
|||
|
Sample matrix |
ID |
Reference |
Reference + 1µl OPG |
% Competition |
|
Heparin plasma |
h1 |
0.31 |
0.00 |
100 |
|
Heparin plasma |
h2 |
0.18 |
0.00 |
100 |
|
Heparin plasma |
h3 |
0.39 |
0.00 |
100 |
|
Heparin plasma |
h4 |
0.49 |
0.00 |
100 |
|
Heparin plasma |
h5 |
0.06 |
0.00 |
100 |
|
Heparin plasma |
h6 |
0.04 |
0.00 |
100 |
|
Heparin plasma |
h7 |
0.19 |
0.01 |
95 |
|
Heparin plasma |
h8 |
0.10 |
0.00 |
100 |
|
Mean |
100 |
|||
Cross-Reactivity
Primates: The sequence homology of human sRANKL (soluble form, aa 140-317) to various primate species is >95%. It is likely that the assay can be used for these species. Internal validations have not been carried out.
Sample Stability
We recommend performing serum or plasma separation by centrifugation as soon as possible, e.g. 20 min at 2000 x g, preferably at 4°C (2-8°C). If this is not possible store the samples at 4°C (2-8°C) prior to centrifugation (up to one day).
The acquired serum or plasma samples should be measured as soon as possible. For longer storage aliquot samples and store at -25°C, for long time storage at -80°C. The stability of endogenous soluble RANKL was tested by comparing RANKL measurements in samples that had undergone 3 freeze-thaw cycles.
Freeze-Thaw Stability
For freeze-thaw experiments, samples were collected according to the supplier’s instruction using blood collection devices and stored at -80°C. Reference samples were freeze-thawed once. The mean recovery of sample concentration in serum after 3 freeze-thaw cycles is 92%.
|
Soluble RANKL c[pmol/l] |
|||||
|
ID |
Reference |
1x |
3x |
CV (%) |
% Recovery after 3 freeze/thaw cycles |
|
s1 |
0.13 |
0.10 |
0.10 |
1 |
76 |
|
s2 |
0.13 |
0.12 |
0.11 |
7 |
87 |
|
s3 |
0.27 |
0.28 |
0.23 |
9 |
87 |
|
s4 |
0.67 |
0.62 |
0.58 |
7 |
86 |
|
s5 |
0.18 |
0.16 |
0.18 |
6 |
95 |
|
s6 |
0.78 |
0.75 |
0.74 |
3 |
94 |
|
s7 |
0.24 |
0.22 |
0.25 |
6 |
104 |
|
s8 |
0.33 |
0.31 |
0.34 |
4 |
103 |
|
s9 |
0.23 |
0.22 |
0.21 |
4 |
92 |
|
Mean |
7 |
92 |
|||
Samples can undergo at least up to 3 freeze-thaw cycles.
Independently, an external CRO determined mean recovery after 4 freeze-thaw cycles to be 102% in serum samples.
|
Soluble RANKL c[pmol/l] |
|
|
||||
|
ID |
Reference (1x) |
2x |
3x |
4x |
CV (%) |
% Recovery after 4 freeze/thaw cycles |
|
s1 |
0.131 |
0.131 |
0.138 |
0.140 |
7 |
107 |
|
s2 |
0.132 |
0.133 |
0.128 |
0.131 |
1 |
99 |
|
s3 |
0.121 |
0.119 |
0.122 |
0.119 |
2 |
98 |
|
s4 |
0.113 |
0.116 |
0.124 |
0.118 |
5 |
105 |
|
s5 |
0.183 |
0.190 |
0.195 |
0.187 |
2 |
102 |
|
s6 |
0.189 |
0.189 |
0.187 |
0.184 |
3 |
97 |
|
|
Mean |
7 |
102 |
|||
Whole Blood Stability
Human serum sample concentrations which were stored for 20h in whole blood at room temperature show a CV of 3%.
Human Heparin plasma sample concentrations which were stored for 20h in whole blood at room temperature show a CV of 6%.
Free sRANKL is stable in whole blood for 20h at room temperature (18-26°C).
Experiment:
Stability of free sRANKL in whole blood was tested in serum and Heparin plasma samples, directly after collection and after 2h, 4h and 20h.
|
|
|
Duration between blood draw and sample prep [h] |
|
|
||||||
|
|
|
0 |
2 |
4 |
20 |
|
|
|||
|
Sample Matrix |
ID |
Soluble RANKL [pmol/l] |
Mean |
% CV |
||||||
|
Serum |
s1 |
0.33 |
0.32 |
0.36 |
0.31 |
0.33 |
6 |
|||
|
Serum |
s2 |
0.33 |
0.33 |
0.33 |
0.33 |
0.33 |
0 |
|||
|
Serum |
s3 |
0.40 |
0.39 |
0.37 |
0.36 |
0.38 |
5 |
|||
|
Serum |
s4 |
0.22 |
0.22 |
0.21 |
0.22 |
0.22 |
2 |
|||
|
|
|
|
|
Mean |
3 |
|||||
|
|
|
Duration between blood draw and sample prep [h] |
|
|
||||||
|
|
|
0 |
2 |
4 |
20 |
|
|
|||
|
Sample Matrix |
ID |
Soluble RANKL [pmol/l] |
Mean |
% CV |
||||||
|
Heparin plasma |
h1 |
0.35 |
0.34 |
0.34 |
0.30 |
0.33 |
7 |
|||
|
Heparin plasma |
h2 |
0.36 |
0.34 |
0.37 |
0.33 |
0.35 |
5 |
|||
|
Heparin plasma |
h3 |
0.39 |
0.39 |
0.40 |
0.38 |
0.39 |
3 |
|||
|
Heparin plasma |
h4 |
0.27 |
0.24 |
0.23 |
0.21 |
0.24 |
10 |
|||
|
|
|
|
|
Mean |
6 |
|||||
Sample Stability at Room Temperature
Serum sample stability at room temperature was assessed by an independent external CRO:
|
Sample ID |
T-0H pmol/l |
T-3H pmol/l |
% variation with T-0H |
T-6H pmol/l |
% variation with T-0H |
T-24H pmol/l |
% variation with T-0H |
|
C1 |
0.130 |
0.134 |
3.1 |
0.133 |
2.3 |
0.125 |
-3.8 |
|
C2 |
0.134 |
0.133 |
-0.7 |
0.134 |
0.0 |
0.127 |
-5.2 |
|
D1 |
0.110 |
0.117 |
6.4 |
0.120 |
9.1 |
0.110 |
0.0 |
|
D2 |
0.124 |
0.119 |
-4.0 |
0.122 |
-1.6 |
0.111 |
-10.5 |
|
E1 |
0.176 |
0.185 |
5.1 |
0.190 |
8.0 |
0.183 |
4.0 |
|
E2 |
0.195 |
0.193 |
-1.0 |
0.193 |
-1.0 |
0.180 |
-7.7 |
At 24 hours 6/6 results have variation <11 %. free sRANKL is stable up to 24 hours at Room Temperature.
Sample Stability at +4°C
Serum sample stability at +4°C was assessed by an independent external CRO:
|
Sample ID |
T-0H pmol/l |
T-3H pmol/l |
% variation with T-0H |
T-6H pmol/l |
% variation with T-0H |
T-24H pmol/l |
% variation with T-0H |
|
C1 |
0.133 |
0.121 |
-9.0 |
0.118 |
-11.3 |
0.116 |
-12.8 |
|
C2 |
0.117 |
0.120 |
2.6 |
0.119 |
1.7 |
0.119 |
1.7 |
|
D1 |
0.110 |
0.111 |
0.9 |
0.109 |
-0.9 |
0.098 |
-10.9 |
|
D2 |
0.107 |
0.106 |
-0.9 |
0.108 |
0.9 |
0.117 |
9.3 |
|
E1 |
0.184 |
0.189 |
2.7 |
0.197 |
7.1 |
0.190 |
3.3 |
|
E2 |
0.192 |
0.193 |
0.5 |
0.194 |
1.0 |
0.193 |
0.5 |
At 24 hours 6/6 results have variation <13 %. free sRANKL is stable up to 24 hours at +4°C.
Sample Values
Free Soluble RANKL Values in Apparently Healthy Individuals
To provide expected values for circulating free soluble RANKL, a panel of samples from apparently healthy donors was tested.
A summary of the results is shown below:
|
|
Soluble RANKL [pmol/l] |
|||
|
Sample Matrix |
n |
Median |
Minimum |
Maximum |
|
Serum |
32 |
0.15 |
0.03 |
0.48 |
|
Heparin plasma |
8 |
0.15 |
0.02 |
0.40 |
It is recommended to establish the normal range for each laboratory.
An apparently healthy cohort (n=88) was also measured by an external CRO:
|
Serum |
Free soluble RANKL [pmol/l] |
|
Mean value |
0.132 |
|
SD |
0.092 |
|
Mean -SD |
0.040 |
|
Mean +SD |
0.224 |
|
n= |
88 |
|
Percentile 5% |
0.025 |
|
Percentile 95% |
0.301 |
|
Percentile 2.5% |
0.021 |
|
Percentile 97.5% |
0.356 |
Screening of 30 sera from apparently healthy men yielded the following results:
|
Serum |
Free soluble RANKL [pmol/l] |
|
Mean value |
0.153 |
|
SD |
0.103 |
|
Mean -SD |
0.050 |
|
Mean +SD |
0.256 |
|
n |
30 |
|
Percentile 5% |
0.026 |
|
Percentile 95% |
0.342 |
|
Percentile 2.5% |
0.022 |
|
Percentile 97.5% |
0.381 |
Screening of 28 sera from apparently healthy pre-menopausal women yielded the following results:
|
Serum |
Free soluble RANKL [pmol/l] |
|
Mean value |
0.121 |
|
SD |
0.089 |
|
Mean - SD |
0.032 |
|
Mean + SD |
0.210 |
|
n |
28 |
|
Percentile 5% |
0.022 |
|
Percentile 95% |
0.282 |
|
Percentile 2.5% |
0.020 |
|
Percentile 97.5% |
0.329 |
Screening of 30 sera from apparently healthy postmenopausal women yielded the following results:
|
Serum |
Free soluble RANKL [pmol/l] |
|
Mean value |
0.123 |
|
SD |
0.081 |
|
Mean -SD |
0.042 |
|
Mean +SD |
0.204 |
|
n |
30 |
|
Percentile 5% |
0.030 |
|
Percentile 95% |
0.266 |
|
Percentile 2.5% |
0.029 |
|
Percentile 97.5% |
0.274 |
Matrix Comparison
To assess whether the two tested matrices behave the same way in the free soluble RANKL ELISA, concentrations of RANKL were measured in serum and heparin plasma samples prepared from 18 apparently healthy donor. Each individual donated blood in both tested sample matrices.
A summary table of soluble RANKL levels in various sample matrices is shown below:
|
Soluble RANKL [pmol/l] |
|
||
|
Sample ID |
Serum |
Heparin plasma |
% CV |
|
#1 |
0.16 |
0.15 |
0 |
|
#2 |
0.20 |
0.23 |
12 |
|
#3 |
0.15 |
0.17 |
12 |
|
#4 |
0.09 |
0.13 |
25 |
|
#5 |
0.07 |
0.09 |
16 |
|
#6 |
0.35 |
0.40 |
9 |
|
#7 |
0.17 |
0.16 |
6 |
|
#8 |
0.17 |
0.16 |
7 |
|
#9 |
0.02 |
0.02 |
14 |
|
#10 |
0.15 |
0.15 |
0 |
|
#11 |
0.08 |
0.10 |
14 |
|
#12 |
0.26 |
0.28 |
5 |
|
#13 |
0.06 |
0.05 |
9 |
|
#14 |
0.20 |
0.18 |
5 |
|
#15 |
0.27 |
0.29 |
5 |
|
#16 |
0.07 |
0.10 |
26 |
|
#17 |
0.15 |
0.19 |
16 |
|
#18 |
0.06 |
0.05 |
5 |
|
|
Mean |
10 |
|
-
Ma, X.-R., Wang, Y., Sun, Y.-C., 2019. Chinese Medical Journal 132, 25.
-
Sclerostin and its association with insulin resistance in children and adolescents.
Wędrychowicz, A., Sztefko, K., Starzyk, J.B., 2019. Bone 120, 232–238.
-
Anagnostis, P., Vakalopoulou, S., Christoulas, D., Paschou, S.A., Papatheodorou, A., Garipidou, V., Kokkoris, P., Terpos, E., 2018. Haemophilia 24, 316–322.
-
Does the Use of a “Walking Bleaching” Technique Increase Bone Resorption Markers?
Bersezio, C., Vildósola, P., Sáez, M., Sánchez, F., Vernal, R., Oliveira, O.B., Jorquera, G., Basualdo, J., Loguercio, A., Fernández, E., 2018. Oper Dent 43, 250–260.
PMID: 29533717
-
Boutsikas, G., Terpos, E., Papatheodorou, A., Tsirkinidis, P., Tsirigotis, P., Meletiou, A., Lalou, E., Telonis, V., Zannou, A., Kanellopoulos, A., Galani, Z., Stefanou, A., Tsaftaridis, P., Viniou, N.-A., Panayiotidis, P., Kyrtsonis, M.-C., Meletis, J., Vassilakopoulos, T.P., Angelopoulou, M.K., 2018. European Journal of Haematology 100, 131–139.
-
Caparbo, V.F., Saad, C.G.S., Moraes, J.C., de Brum-Fernandes, A.J., Pereira, R.M.R., 2018. Osteoporos Int.
PMID: 30006885
-
Davenport, C., Harper, E., Rochfort, K.D., Forde, H., Smith, D., Cummins, P.M., 2018. J. Vasc. Res. 55, 111–123.
PMID: 29635231
-
Eleutherakis-Papaiakovou, E., Kastritis, E., Gavriatopoulou, M., Christoulas, D., Roussou, M., Ntanasis-Stathopoulos, I., Kanellias, N., Papatheodorou, A., Dimopoulos, M.A., Terpos, E., 2018. Clin Lymphoma Myeloma Leuk 18, 431–437.
PMID: 29685422
-
Evenepoel, P., Claes, K., Meijers, B., Laurent, M., Bammens, B., Naesens, M., Sprangers, B., Pottel, H., Cavalier, E., Kuypers, D., 2018. J. Bone Miner. Res.
PMID: 30427544
-
Vitamin D status, estradiol and bone metabolism in elderly female patients with hip fracture.
Fakler, J.K.M., 2018. International Journal of Orthopaedics 5, 876-882–882.
-
Glasnović, A., Stojić, M., Dežmalj, L., Tudorić-Đeno, I., Romić, D., Jeleč, V., Vrca, A., Vuletić, V., Grčević, D., 2018. Neuroimmunomodulation 25, 23–33.
PMID: 29920500
-
Grzelak, T., Wedrychowicz, A., Grupinska, J., Pelczynska, M., Sperling, M., Mikulska, A.A., Naughton, V., Czyzewska, K., 2018. Arch Med Sci 14.
-
Jansen, R.B., Christensen, T.M., Bülow, J., Rørdam, L., Holstein, P.E., Jørgensen, N.R., Svendsen, O.L., 2018. J. Diabetes Complicat. 32, 164–170.
PMID: 29196119
-
Kardos, Z., Oláh, C., Sepsi, M., Sas, A., Kostyál, L., Bóta, T., Bhattoa, H.P., Hodosi, K., Kerekes, G., Tamási, L., Bereczki, D., Szekanecz, Z., 2018. Clin. Rheumatol. 37, 1183–1188.
PMID: 29383454
-
Mantovani, A., Sani, E., Fassio, A., Colecchia, A., Viapiana, O., Gatti, D., Idolazzi, L., Rossini, M., Salvagno, G., Lippi, G., Zoppini, G., Byrne, C.D., Bonora, E., Targher, G., 2018. Diabetes Metab.
PMID: 30315891
-
Relation of RANKL and OPG Levels with Bone Resorption in Patients with Acromegaly and Prolactinoma.
Ozer, F.F., Dagdelen, S., Erbas, T., 2018. Horm. Metab. Res. 50, 562–567.
PMID: 29895074
-
Rachner, T.D., Kasimir-Bauer, S., Göbel, A., Erdmann, K., Hoffmann, O., Browne, A.J., Wimberger, P., Rauner, M., Hofbauer, L.C., Kimmig, R., Bittner, A.-K., 2018. Clin. Cancer Res.
PMID: 30425091
-
Sarink, D., Schock, H., Johnson, T., Chang-Claude, J., Overvad, K., Olsen, A., Tjønneland, A., Arveux, P., Fournier, A., Kvaskoff, M., Boeing, H., Karakatsani, A., Trichopoulou, A., La Vecchia, C., Masala, G., Agnoli, C., Panico, S., Tumino, R., Sacerdote, C., van Gils, C.H., Peeters, P.H.M., Weiderpass, E., Agudo, A., Rodríguez-Barranco, M., Huerta, J.M., Ardanaz, E., Gil, L., Kaw, K.T., Schmidt, J.A., Dossus, L., His, M., Aune, D., Riboli, E., Kaaks, R., Fortner, R.T., 2018. BMC Cancer 18, 1010.
PMID: 30348163; PMCID: PMC6196438
-
sRANKL and its correlation with metabolic syndrome parameters in children.
Serrano-Piña, R., Trujillo-Güiza, M.L., Scougall Vilchis, R.J., Layton-Tovar, C.F., Mendieta-Zerón, H., 2018. Int J Paediatr Dent.
PMID: 30252176
-
Sewerin, P., Le, L., Vordenbäumen, S., Schleich, C., Sengewein, R., Brinks, R., Pongratz, G., Bleck, E., Lesch, J., Mansmann, U., Schneider, M., Ostendorf, B., 2018. The Journal of Rheumatology 45, 753–759.
-
Terpos, E., Kastritis, E., Ntanasis-Stathopoulos, I., Christoulas, D., Papatheodorou, A., Eleutherakis-Papaiakovou, E., Kanellias, N., Fotiou, D., Ziogas, D.C., Migkou, M., Roussou, M., Trougkakos, I.P., Gavriatopoulou, M., Dimopoulos, M.A., 2018. Am. J. Hematol.
PMID: 30592079
-
Tsirkinidis, P., Terpos, E., Boutsikas, G., Papatheodorou, A., Anargyrou, K., Lalou, E., Dimitrakopoulou, A., Kalpadakis, C., Konstantopoulos, K., Siakantaris, M., Panayiotidis, P., Pangalis, G., Kyrtsonis, M.-C., Vassilakopoulos, T., Angelopoulou, M.K., 2018. Journal of Bone and Mineral Metabolism 36, 399–409.
-
Caspase-mediated Apoptosis by Compressive Force Induces RANKL in Cementoblasts.
Yamaguchi, M., Minato, Y., Shimizu, M., Kikuta, J., Hikida, T., Kasai, K., 2018. IJOMS 16, 31–38.
-
Zimmermann, A., Popp, R.A., Rossmann, H., Bucerzan, S., Nascu, I., Leucuta, D., Weber, M.M., Grigorescu-Sido, P., 2018. Therapeutics and Clinical Risk Management Volume 14, 2069–2080.
-
Bruhn-Olszewska, B., Korzon-Burakowska, A., Węgrzyn, G., Jakóbkiewicz-Banecka, J., 2017. Sci Rep 7, 501.
PMID: 28356555; PMCID: PMC5428699
-
Markers of bone metabolism during 14 days of bed rest in young and older men.
Buehlmeier, J., Frings-Meuthen, P., Mohorko, N., Lau, P., Mazzucco, S., Ferretti, J.L., Biolo, G., Pisot, R., Simunic, B., Rittweger, J., 2017 10
-
Biochemical markers of bone metabolism during early orthodontic tooth movement with aligners.
Castroflorio, T., Gamerro, E.F., Caviglia, G.P., Deregibus, A., 2017. Angle Orthod 87, 74–81.
PMID: 27409364
-
Osteoprotegerin, RANKL, ADMA, and Fetuin-A serum levels in children with type I diabetes mellitus.
Chrysis, D., Efthymiadou, A., Mermigka, A., Kritikou, D., Spiliotis, B.E., 2017. Pediatr Diabetes 18, 277–282.
PMID: 27028343
-
Gifre, L., Ruiz-Gaspà, S., Carrasco, J.L., Portell, E., Vidal, J., Muxi, A., Monegal, A., Guañabens, N., Peris, P., 2017. Osteoporos Int 28, 2707–2715.
PMID: 28580511
-
Loss of Functional Osteoprotegerin: More Than a Skeletal Problem.
Grasemann, C., Unger, N., Hövel, M., Arweiler-Harbeck, D., Herrmann, R., Schündeln, M.M., Müller, O., Schweiger, B., Lausch, E., Meissner, T., Kiewert, C., Hauffa, B.P., Shaw, N.J., 2017. J. Clin. Endocrinol. Metab. 102, 210–219.
PMID: 27809640
-
Hauser, B., Zhao, S., Visconti, M.R., Riches, P.L., Fraser, W.D., Piec, I., Goodson, N.J., Ralston, S.H., 2017. Calcif. Tissue Int. 101, 375–383.
PMID: 28534161; PMCID: PMC5587630
-
NOTCH1 Mutations in Aortic Stenosis: Association with Osteoprotegerin/RANK/RANKL.
Irtyuga, O., Malashicheva, A., Zhiduleva, E., Freylikhman, O., Rotar, O., Bäck, M., Tarnovskaya, S., Kostareva, A., Moiseeva, O., 2017. Biomed Res Int 2017, 6917907.
PMID: 28246602; PMCID: PMC5299165
-
Kushlinskii, N.E., Gershtein, E.S., Solov’ev, Y.N., Timofeev, Y.S., Babkina, I.V., Dolinkin, A.O., Zuev, A.A., Kostyleva, O.I., 2017. Bull. Exp. Biol. Med. 163, 478–481.
PMID: 28853064
-
Lambert, M.N.T., Thybo, C.B., Lykkeboe, S., Rasmussen, L.M., Frette, X., Christensen, L.P., Jeppesen, P.B., 2017. Am. J. Clin. Nutr. 106, 909–920.
PMID: 28768651
-
Weekly oral bisphosphonates over 2 years prevent bone loss in cardiac transplant patients.
Lange, U., Classen, K., Müller-Ladner, U., Richter, M., 2017. Clin Transplant 31.
PMID: 28940569
-
Effects of short-term dry immersion on bone remodeling markers, insulin and adipokines.
Linossier, M.-T., Amirova, L.E., Thomas, M., Normand, M., Bareille, M.-P., Gauquelin-Koch, G., Beck, A., Costes-Salon, M.-C., Bonneau, C., Gharib, C., Custaud, M.-A., Vico, L., 2017. PLOS ONE 12, e0182970.
-
Martín-Saavedra, F., Crespo, L., Escudero-Duch, C., Saldaña, L., Gómez-Barrena, E., Vilaboa, N., 2017. Sci Rep 7, 15182.
PMID: 29123118; PMCID: PMC5680323
-
Long-Term Effects of Severe Burn Injury on Bone Turnover and Microarchitecture.
Muschitz, G.K., Schwabegger, E., Fochtmann, A., Baierl, A., Kocijan, R., Haschka, J., Gruther, W., Schanda, J.E., Resch, H., Rath, T., Pietschmann, P., Muschitz, C., 2017. Journal of Bone and Mineral Research 32, 2381–2393.
-
Effect of Tumor Necrosis Factor Inhibitor Therapy on Osteoclasts Precursors in Rheumatoid Arthritis.
Perpétuo, Inês P., Caetano-Lopes, J., Rodrigues, A.M., Campanilho-Marques, R., Ponte, C., Canhão, H., Ainola, M., Fonseca, J.E., 2017. BioMed Research International, 1–10.
-
Perpétuo, Inês Pedro, Caetano-Lopes, J., Rodrigues, A.M., Campanilho-Marques, R., Ponte, C., Canhão, H., Ainola, M., Fonseca, J.E., 2017. RMD Open 3, e000365.
PMID: 28955481; PMCID: PMC5604603
-
Perpétuo, Inês P., Caetano-Lopes, J., Vieira-Sousa, E., Campanilho-Marques, R., Ponte, C., Canhão, H., Ainola, M., Fonseca, J.E., 2017. Frontiers in Medicine 4.
-
Saldaña, L., Vallés, G., Bensiamar, F., Mancebo, F.J., García-Rey, E., Vilaboa, N., 2017. Sci Rep 7, 14618.
PMID: 29097745; PMCID: PMC5668416
-
Sarink, D., Schock, H., Johnson, T., Overvad, K., Holm, M., Tjønneland, A., Boutron-Ruault, M.-C., His, M., Kvaskoff, M., Boeing, H., Lagiou, P., Papatesta, E.-M., Trichopoulou, A., Palli, D., Pala, V., Mattiello, A., Tumino, R., Sacerdote, C., Bueno-de-Mesquita, H.B.A., van Gils, C.H., Peeters, P.H., Weiderpass, E., Agudo, A., Sánchez, M.-J., Chirlaque, M.-D., Ardanaz, E., Amiano, P., Khaw, K.T., Travis, R., Dossus, L., Gunter, M., Rinaldi, S., Merritt, M., Riboli, E., Kaaks, R., Fortner, R.T., 2017. Cancer Prev Res 10, 525–534.
PMID: 28701332; PMCID: PMC5603271
-
Serum irisin and myostatin levels after 2 weeks of high-altitude climbing.
Śliwicka, E., Cisoń, T., Kasprzak, Z., Nowak, A., Pilaczyńska-Szcześniak, Ł., 2017. PLoS ONE 12, e0181259.
PMID: 28732027; PMCID: PMC5521782
-
Atypical skeletal manifestations of rickets in a familial hypocalciuric hypercalcemia patient.
Wu, B., Wang, O., Jiang, Y., Li, M., Xing, X., Xia, W., 2017. Bone Res 5, 17001.
PMID: 28690912; PMCID: PMC5486235
-
Hajdu Cheney Syndrome; report of a novel NOTCH2 mutation and treatment with denosumab.
Adami, G., Rossini, M., Gatti, D., Orsolini, G., Idolazzi, L., Viapiana, O., Scarpa, A., Canalis, E., 2016. Bone 92, 150–156.
PMID: 27592446; PMCID: PMC5056853
-
Chhana, A., Aati, O., Gamble, G.D., Callon, K.E., Doyle, A.J., Roger, M., McQueen, F.M., Horne, A., Reid, I.R., Cornish, J., Dalbeth, N., 2016. The Journal of Rheumatology 43, 445–449.
-
Fonolla-Joya, J., Reyes-García, R., García-Martín, A., López-Huertas, E., Muñoz-Torres, M., 2016. J Am Coll Nutr 35, 529–536.
PMID: 27463412
-
Gómez-Vaquero, C., Martín, I., Loza, E., Carmona, L., Ivorra, J., Narváez, J.A., Hernández-Gañán, J., Alía, P., Narváez, J., 2016. PLOS ONE 11, e0166691.
-
Aberrant regulation of RANKL/OPG in women at high risk of developing breast cancer.
Kiechl, S., Schramek, D., Widschwendter, M., Fourkala, E.-O., Zaikin, A., Jones, A., Jaeger, B., Rack, B., Janni, W., Scholz, C., Willeit, J., Weger, S., Mayr, A., Teschendorff, A., Rosenthal, A., Fraser, L., Philpott, S., Dubeau, L., Keshtgar, M., Roylance, R., Jacobs, I.J., Menon, U., Schett, G., Penninger, J.M., 2016. Oncotarget 8, 3811–3825.
PMID: 28002811; PMCID: PMC5354797
-
Melchiorre, D., Linari, S., Manetti, M., Romano, E., Sofi, F., Matucci-Cerinic, M., Carulli, C., Innocenti, M., Ibba-Manneschi, L., Castaman, G., 2016. Haematologica 101, 219–225.
PMID: 26494839; PMCID: PMC4938330
-
Nakahara, T., Kawai-Kowase, K., Matsui, H., Sunaga, H., Utsugi, T., Iso, T., Arai, M., Tomono, S., Kurabayashi, M., 2016. Atherosclerosis 253, 102–110.
PMID: 27599364
-
Oster, M., Just, F., Büsing, K., Wolf, P., Polley, C., Vollmar, B., Muráni, E., Ponsuksili, S., Wimmers, K., 2016. Am. J. Physiol. Regul. Integr. Comp. Physiol. 310, R917-925.
PMID: 26962023; PMCID: PMC4896080
-
Palma, B.D., Guasco, D., Pedrazzoni, M., Bolzoni, M., Accardi, F., Costa, F., Sammarelli, G., Craviotto, L., De Filippo, M., Ruffini, L., Omedè, P., Ria, R., Aversa, F., Giuliani, N., 2016. Leukemia 30, 409–416.
-
Pereira, R.M.R., Figueiredo, C.P., Cha, C.C., Caparbo, V.F., Oliveira, R.M., Franco, A.S., Menezes, P.R., de Castro, I., Onuchic, L.F., 2016. Osteoporos Int 27, 3319–3329.
PMID: 27311721
-
Expression of osteoprotegerin and its ligands, RANKL and TRAIL, in rheumatoid arthritis.
Remuzgo-Martínez, S., Genre, F., López-Mejías, R., Ubilla, B., Mijares, V., Pina, T., Corrales, A., Blanco, R., Martín, J., Llorca, J., González-Gay, M.A., 2016. Sci Rep 6, 29713.
PMID: 27403809; PMCID: PMC4940734
-
Hormone concentrations throughout uncomplicated pregnancies: a longitudinal study.
Schock, H., Zeleniuch-Jacquotte, A., Lundin, E., Grankvist, K., Lakso, H.-Å., Idahl, A., Lehtinen, M., Surcel, H.-M., Fortner, R.T., 2016. BMC Pregnancy Childbirth 16, 146.
PMID: 27377060; PMCID: PMC4932669
-
Differences in biochemical bone markers by diabetes type and the impact of glucose.
Starup-Linde, J., Lykkeboe, S., Gregersen, S., Hauge, E.-M., Langdahl, B.L., Handberg, A., Vestergaard, P., 2016. Bone 83, 149–155.
PMID: 26555635
-
Stuss, M., Sewerynek, E., Król, I., Stępień-Kłos, W., Jędrzejczyk, S., 2016. Endokrynol Pol 67, 174–184.
PMID: 26884284
-
Tohyama, R., Kayamori, K., Sato, K., Hamagaki, M., Sakamoto, K., Yasuda, H., Yamaguchi, A., 2016. J. Oral Pathol. Med. 45, 356–364.
PMID: 26859422
-
Ugay, L., Kochetkova, E., Nevzorova, V., Maistrovskaia, Y., 2016. Chin. Med. J. 129, 1696–1703.
PMID: 27411457; PMCID: PMC4960959
-
Uluçkan, Ö., Jimenez, M., Karbach, S., Jeschke, A., Graña, O., Keller, J., Busse, B., Croxford, A.L., Finzel, S., Koenders, M., van den Berg, W., Schinke, T., Amling, M., Waisman, A., Schett, G., Wagner, E.F., 2016. Sci Transl Med 8, 330ra37.
PMID: 27089206
-
Craniometaphyseal dysplasia with obvious biochemical abnormality and rickets-like features.
Wu, B., Jiang, Y., Wang, O., Li, M., Xing, X.-P., Xia, W.-B., 2016. Clin. Chim. Acta 456, 122–127.
PMID: 26820766
-
Anastasilakis, Athanasios D., Polyzos, S.A., Efstathiadou, Z.A., Savvidis, M., Sakellariou, G.T., Papatheodorou, A., Kokkoris, P., Makras, P., 2015. Metab. Clin. Exp. 64, 1291–1297.
PMID: 26198440
-
Denosumab versus zoledronic acid in patients previously treated with zoledronic acid.
Anastasilakis, A. D., Polyzos, S.A., Gkiomisi, A., Saridakis, Z.G., Digkas, D., Bisbinas, I., Sakellariou, G.T., Papatheodorou, A., Kokkoris, P., Makras, P., 2015. Osteoporos Int 26, 2521–2527.
PMID: 25990355
-
Asadipooya, K., Graves, L., Lukert, B.P., Kalantarhormozi, M., Assadi, M., Ostovar, A., Larijani, B., Nabipour, I., 2015. Mediterranean Journal of Nutrition and Metabolism 8, 231–241.
-
Bonfá, A.C., Seguro, L.P.C., Caparbo, V., Bonfá, E., Pereira, R.M.R., 2015. Osteoporos Int 26, 1563–1571.
PMID: 25609157
-
Polymorphisms in neuropeptide genes and bone mineral density in Korean postmenopausal women.
Chun, E.H., Kim, H., Suh, C.S., Kim, J.H., Kim, D.Y., Kim, J.G., 2015. Menopause 22, 1256–1263.
PMID: 25871004
-
Demoulin, S.A., Somja, J., Duray, A., Guénin, S., Roncarati, P., Delvenne, P.O., Herfs, M.F., Hubert, P.M., 2015. Oncoimmunology 4, e1008334.
PMID: 26155412; PMCID: PMC4485731
-
Mechanisms of enhanced osteoclastogenesis in girls and young women with Turner’s Syndrome.
Faienza, M.F., Brunetti, G., Ventura, A., Piacente, L., Messina, M.F., De Luca, F., Ciccarelli, M., Oranger, A., Mori, G., Natale, M.P., Gigante, M., Ranieri, E., Gesualdo, L., Colucci, S., Cavallo, L., Grano, M., 2015. Bone 81, 228–236.
PMID: 26208797
-
Gaudio, A., Muratore, F., Fiore, V., Rapisarda, R., Signorelli, S.S., Fiore, C.E., 2015. Osteoporos Int 26, 1747–1753.
PMID: 25672808
-
Ghorbanihaghjo, A., Argani, H., Rashtchizadeh, N., Raeisi, S., Rashadi, J., Nourani nia, S., 2015. Ind J Clin Biochem 30, 430–434.
-
Jasiewicz, M., Knapp, M., Waszkiewicz, E., Ptaszynska-Kopczynska, K., Szpakowicz, A., Sobkowicz, B., Musial, W.J., Kaminski, K.A., 2015. Cytokine 76, 187–192.
PMID: 26163998
-
Kastritis, E., Gavriatopoulou, M., Dimopoulos, M.A., Eleutherakis-Papaiakovou, E., Kanellias, N., Roussou, M., Pamboucas, C., Toumanidis, S.T., Terpos, E., 2015. Blood Cancer J 5, e319.
PMID: 26047389; PMCID: PMC4648482
-
Notch signaling induces root resorption via RANKL and IL-6 from hPDL cells.
Kikuta, J., Yamaguchi, M., Shimizu, M., Yoshino, T., Kasai, K., 2015. J. Dent. Res. 94, 140–147.
PMID: 25376720
-
Kulcsar-Jakab, E., Petho, Z., Pap, Z., Kalina, E., Foldesi, R., Balogh, A., Antal-Szalmas, P., Bhattoa, H.P., 2015. BMC Musculoskelet Disord 16, 227.
PMID: 26311162; PMCID: PMC4551745
-
Kurz, K., Herold, M., Russe, E., Klotz, W., Weiss, G., Fuchs, D., 2015. Dis. Markers, 276969.
PMID: 26576067; PMCID: PMC4631883
-
Negredo, E., Diez-Pérez, A., Bonjoch, A., Domingo, P., Pérez-Álvarez, N., Gutierrez, M., Mateo, G., Puig, J., Echeverría, P., Escrig, R., Clotet, B., 2015. J. Antimicrob. Chemother. 70, 2104–2107.
PMID: 25769303
-
Onuma, T., Aquiar, K., Duarte, P.M., Feres, M., Giro, G., Coelho, P., Cassoni, A., Shibli, J.A., 2015. Int J Oral Maxillofac Implants 30, 1431–1436.
PMID: 26478977
-
Ostrowska, Z., Ziora, K., Oświęcimska, J., Marek, B., Świętochowska, E., Kajdaniuk, D., Strzelczyk, J., Cieślicka, A., Wołkowska-Pokrywa, K., Kos-Kudła, B., 2015. Endokrynol Pol 66, 313–321.
PMID: 26323468
-
Pitari, M.R., Rossi, M., Amodio, N., Botta, C., Morelli, E., Federico, C., Gullà, A., Caracciolo, D., Di Martino, M.T., Arbitrio, M., Giordano, A., Tagliaferri, P., Tassone, P., 2015. Oncotarget 6, 27343–27358.
PMID: 26160841; PMCID: PMC4694994
-
Rakic, M., Petkovic-Curcin, A., Struillou, X., Matic, S., Stamatovic, N., Vojvodic, D., 2015. Clin Oral Investig 19, 791–801.
PMID: 25217276
-
Seguro, L.P.C., Casella, C.B., Caparbo, V.F., Oliveira, R.M., Bonfa, A., Bonfa, E., Pereira, R.M.R., 2015. Osteoporosis International 26, 459–467.
-
Telejko, B., Kalejta, K., Kuzmicki, M., Wawrusiewicz-Kurylonek, N., Lipinska, D., Pliszka, J., Wilk, J., Zielinska, A., Sobota, A., Szamatowicz, J., Kretowski, A., Gorska, M., 2015. Ann Agric Environ Med 22, 307–312.
PMID: 26094529
-
Topographical cues regulate the crosstalk between MSCs and macrophages.
Vallés, G., Bensiamar, F., Crespo, L., Arruebo, M., Vilaboa, N., Saldaña, L., 2015. Biomaterials 37, 124–133.
PMID: 25453943; PMCID: PMC4245715
-
Cannabinoid receptor gene polymorphisms and bone mineral density in Korean postmenopausal women.
Woo, J.H., Kim, H., Kim, J.H., Kim, J.G., 2015. Menopause 22, 512–519.
PMID: 25268406
-
Serum sclerostin in high-activity adult patients with juvenile idiopathic arthritis.
Brabnikova-Maresova, K., Jarosova, K., Pavelka, K., Stepan, J.J., 2014. Arthritis Research & Therapy 16.
-
Osteoclastogenic potential of peripheral blood mononuclear cells in cleidocranial dysplasia.
Faienza, M.F., Ventura, A., Piacente, L., Ciccarelli, M., Gigante, M., Gesualdo, L., Colucci, S., Cavallo, L., Grano, M., Brunetti, G., 2014. Int J Med Sci 11, 356–364.
PMID: 24578613; PMCID: PMC3936030
-
Kawashiri, S., Suzuki, T., Nakashima, Y., Horai, Y., Okada, A., Iwamoto, N., Ichinose, K., Tamai, M., Arima, K., Nakamura, H., Origuchi, T., Uetani, M., Aoyagi, K., Eguchi, K., Kawakami, A., 2014. Rheumatology 53, 562–569.
PMID: 24319104
-
Koura, H.M., Zaki, S.M., Ismail, N.A., Salama, E.E., El Lebedy, D.H., Effat, L.K., 2014. Iran J Pediatr 24, 23–28
PMID: 25793041; PMCID: PMC4359600
-
Lim, M.J., Kwon, S.R., Joo, K., Son, M.J., Park, S.-G., Park, W., 2014. The Korean Journal of Internal Medicine 29, 807.
-
Metwalley, K.A., El-Saied, A.-R.A.-H., 2014. Indian J Endocrinol Metab 18, 700–704.
PMID: 25285289; PMCID: PMC4171895
-
Simpson, C.A., Foer, D., Lee, G.S., Bihuniak, J., Sun, B., Sullivan, R., Belsky, J., Insogna, K.L., 2014. Osteoporosis International 25, 2383–2388.
-
Terpos, Evangelos, Christoulas, D., Kastritis, E., Katodritou, E., Papatheodorou, A., Pouli, A., Kyrtsonis, M.-C., Michalis, E., Papanikolaou, X., Gkotzamanidou, M., Koulieris, E., Gavriatopoulou, M., Zervas, K., Dimopoulos, M.A., on behalf of the Greek Myeloma Study Group, 2014. American Journal of Hematology 89, 34–40.
-
Terpos, E, Christoulas, D., Kastritis, E., Roussou, M., Migkou, M., Eleutherakis-Papaiakovou, E., Gavriatopoulou, M., Gkotzamanidou, M., Kanellias, N., Manios, E., Papadimitriou, C., Dimopoulos, M.A., 2014. Leukemia 28, 928–934.
-
Anastasilakis, A.D., Polyzos, S.A., Gkiomisi, A., Bisbinas, I., Gerou, S., Makras, P., 2013. The Journal of Clinical Endocrinology & Metabolism 98, 3206–3212.
-
Traditional and novel bone remodeling markers in premenopausal and postmenopausal women.
Botella, S., Restituto, P., Monreal, I., Colina, I., Calleja, A., Varo, N., 2013. J. Clin. Endocrinol. Metab. 98, E1740-1748.
PMID: 24001743
-
Brunetti, G., Faienza, M.F., Piacente, L., Ventura, A., Oranger, A., Carbone, C., Benedetto, A.D., Colaianni, G., Gigante, M., Mori, G., Gesualdo, L., Colucci, S., Cavallo, L., Grano, M., 2013. American Journal of Physiology-Endocrinology and Metabolism 304, E546–E554.
-
Human apolipoprotein E isoforms differentially affect bone mass and turnover in vivo.
Dieckmann, M., Beil, F.T., Mueller, B., Bartelt, A., Marshall, R.P., Koehne, T., Amling, M., Ruether, W., Cooper, J.A., Humphries, S.E., Herz, J., Niemeier, A., 2013. J. Bone Miner. Res. 28, 236–245.
PMID: 22991192; PMCID: PMC3547162
-
Engvall, I.-L., Svensson, B., Boonen, A., van der Heijde, D., Lerner, U.H., Hafström, I., BARFOT study group, 2013. Rheumatology 52, 733–742.
PMID: 23275387
-
Galeone, A., Brunetti, G., Rotunno, C., Oranger, A., Colucci, S., de Luca Tupputi Schinosa, L., Zallone, A., Grano, M., Paparella, D., 2013. Eur J Cardiothorac Surg 44, e141-147.
PMID: 23671202
-
LaCroix, A.Z., Jackson, R.D., Aragaki, A., Kooperberg, C., Cauley, J.A., Chen, Z., Leboff, M.S., Duggan, D., Wactawski-Wende, J., 2013. Bone 56, 474–481.
PMID: 23735608; PMCID: PMC3832355
-
Liu, J., Zhao, H., Zhao, L., Chen, Y., Zhang, L., Tao, B., Sun, L., Zhao, Y., Wang, W., Xu, M., Chen, J., Ning, G., 2013. J. Clin. Endocrinol. Metab. 98, 2146–2152.
PMID: 23553865
-
Mendonça, M.L., Pereira, F.A., Nogueira-Barbosa, M.H., Monsignore, L.M., Teixeira, S.R., Watanabe, P.C., Maciel, L.M., de Paula, F.J., 2013. BMC Endocr Disord 13, 1.
PMID: 23286605; PMCID: PMC3546901
-
Picarda, G., Matous, E., Amiaud, J., Charrier, C., Lamoureux, F., Heymann, M.-F., Tirode, F., Pitard, B., Trichet, V., Heymann, D., Redini, F., 2013. J Bone Oncol 2, 95–104.
PMID: 26909278; PMCID: PMC4723385
-
Revu, S., Neregård, P., af Klint, E., Korotkova, M., Catrina, A.I., 2013. Arthritis Res. Ther. 15, R205.
PMID: 24295447; PMCID: PMC3978873
-
Saki, F., Karamizadeh, Z., Nasirabadi, S., Mumm, S., McAlister, W.H., Whyte, M.P., 2013. J. Bone Miner. Res. 28, 1501–1508.
PMID: 23322328; PMCID: PMC3663917
-
Schmiedel, B.J., Nuebling, T., Steinbacher, J., Malinovska, A., Wende, C.M., Azuma, M., Schneider, P., Grosse-Hovest, L., Salih, H.R., 2013. J. Immunol. 190, 821–831.
PMID: 23241893
-
Bone metabolism compensates for the delayed growth in small for gestational age neonates.
Tenta, R., Bourgiezi, I., Aliferis, E., Papadopoulou, M., Gounaris, A., Skouroliakou, M., 2013. Organogenesis 9, 55–59.
PMID: 23538775; PMCID: PMC3674041
-
Physical training increases osteoprotegerin in postmenopausal women.
Bergström, I., Parini, P., Gustafsson, S.A., Andersson, G., Brinck, J., 2012. J. Bone Miner. Metab. 30, 202–207.
PMID: 21823052
-
Soluble rank ligand produced by myeloma cells causes generalised bone loss in multiple myeloma.
Buckle, C.H., De Leenheer, E., Lawson, M.A., Yong, K., Rabin, N., Perry, M., Vanderkerken, K., Croucher, P.I., 2012. PLoS ONE 7, e41127.
PMID: 22952578; PMCID: PMC3430669
-
Josse, A.R., Atkinson, S.A., Tarnopolsky, M.A., Phillips, S.M., 2012. J. Clin. Endocrinol. Metab. 97, 251–260.
PMID: 22049177
-
Kaneko, K., Kusunoki, N., Hasunuma, T., Kawai, S., 2012. J. Clin. Endocrinol. Metab. 97, E1909-1917.
PMID: 22791764; PMCID: PMC3462941
-
First inducible transgene expression in porcine large animal models.
Klymiuk, N., Böcker, W., Schönitzer, V., Bähr, A., Radic, T., Fröhlich, T., Wünsch, A., Keßler, B., Kurome, M., Schilling, E., Herbach, N., Wanke, R., Nagashima, H., Mutschler, W., Arnold, G.J., Schwinzer, R., Schieker, M., Wolf, E., 2012. FASEB J. 26, 1086–1099.
PMID: 22138035
-
Serum Osteoprotegerin, RANKL, and Dkk-1 Levels in Adults with Langerhans Cell Histiocytosis.
Makras, P., Polyzos, S.A., Anastasilakis, A.D., Terpos, E., Kanakis, G., Schini, M., Papatheodorou, A., Kaltsas, G.A., 2012. The Journal of Clinical Endocrinology & Metabolism 97, E618–E621.
-
RANK-RANKL-OPG in hemophilic arthropathy: from clinical and imaging diagnosis to histopathology.
Melchiorre, D., Milia, A.F., Linari, S., Romano, E., Benelli, G., Manetti, M., Guiducci, S., Ceccarelli, C., Innocenti, M., Carulli, C., Civinini, R., Morfini, M., Matucci-Cerinic, M., Ibba-Manneschi, L., 2012. J. Rheumatol. 39, 1678–1686.
PMID: 22753650
-
Nagashima, M., Takahashi, H., Shimane, K., Nagase, Y., Wauke, K., 2012. Arthritis Res. Ther. 14, R224.
PMID: 23079134; PMCID: PMC3580535
-
Serum sclerostin increases in healthy adult men during bed rest.
Spatz, J.M., Fields, E.E., Yu, E.W., Divieti Pajevic, P., Bouxsein, M.L., Sibonga, J.D., Zwart, S.R., Smith, S.M., 2012. J. Clin. Endocrinol. Metab. 97, E1736-1740.
PMID: 22767636; PMCID: PMC3431567
-
Tohidi, M., Akbarzadeh, S., Larijani, B., Kalantarhormozi, M., Ostovar, A., Assadi, M., Vahdat, K., Farrokhnia, M., Sanjdideh, Z., Amirinejad, R., Nabipour, I., 2012. Bone 51, 876–881.
PMID: 22971441
-
Vázquez, M.A., Lopez, E., Montoya, M.J., Giner, M., Pérez-Temprano, R., Pérez-Cano, R., 2012. BMC Gastroenterol 12, 47.
PMID: 22584049; PMCID: PMC3438096
-
Zupan, J., Komadina, R., Marc, J., 2012. J. Biomed. Sci. 19, 28.
PMID: 22380539; PMCID: PMC3307025
-
Assadi, M., Salimipour, H., Akbarzadeh, S., Nemati, R., Jafari, S.M., Bargahi, A., Samani, Z., Seyedabadi, M., Sanjdideh, Z., Nabipour, I., 2011. PLoS ONE 6, e24240.
PMID: 21935388; PMCID: PMC3174149
-
Colombini, A., Lombardi, G., Galliera, E., Dogliotti, G., Randelli, P., Meerssemann, A., Mineo, G., Cabitza, P., Corsi, M.M., 2011. Int Orthop 35, 777–782.
PMID: 20623281; PMCID: PMC3080502
-
Serum osteoprotegerin levels are related to height loss: the Tromsø Study.
Jørgensen, L., Hansen, J.-B., Brox, J., Mathiesen, E., Vik, A., Jacobsen, B.K., 2011. Eur. J. Epidemiol. 26, 305–312.
PMID: 21331661; PMCID: PMC3088831
-
Ndip, A., Williams, A., Jude, E.B., Serracino-Inglott, F., Richardson, S., Smyth, J.V., Boulton, A.J.M., Alexander, M.Y., 2011. Diabetes 60, 2187–2196.
PMID: 21659498; PMCID: PMC3142088
-
Pepene, C.E., Ilie, I.R., Marian, I., Duncea, I., 2011. Eur. J. Endocrinol. 164, 61–68.
PMID: 20974706
-
Rauner, M., Goettsch, C., Stein, N., Thiele, S., Bornhaeuser, M., De Bosscher, K., Haegeman, G., Tuckermann, J., Hofbauer, L.C., 2011. Endocrinology 152, 103–112.
PMID: 21084452
-
Stavroulopoulos, A., Porter, C.J., Pointon, K., Monaghan, J.M., Roe, S.D., Cassidy, M.J.D., 2011. Nephrol. Dial. Transplant. 26, 2582–2589.
PMID: 21224493
-
Terpos, E., Fragiadaki, K., Konsta, M., Bratengeier, C., Papatheodorou, A., Sfikakis, P.P., 2011. Clin. Exp. Rheumatol. 29, 921–925
PMID: 22032557
-
Yuan, L.-Q., Zhu, J.-H., Wang, H.-W., Liang, Q.-H., Xie, H., Wu, X.-P., Zhou, H., Cui, R.-R., Sheng, Z.-F., Zhou, H.-D., Zhu, X., Liu, G.-Y., Liu, Y.-S., Liao, E.-Y., 2011. PLoS ONE 6, e29037.
PMID: 22194983; PMCID: PMC3240644
-
Abd El Dayem, S.M., Anwar, G.M., Salama, H., Kamel, A.F., Emara, N., 2010. Arch Med Sci 6, 104–110.
PMID: 22371729; PMCID: PMC3278952
-
Camozzi, V., Sanguin, F., Albigier, N., Scaroni, C., Mantero, F., Zaninotto, M., Frigo, A., Piccolo, M., Luisetto, G., 2010. Eur. J. Endocrinol. 162, 85–90.
PMID: 19793762
-
Dalbeth, N., Pool, B., Smith, T., Callon, K.E., Lobo, M., Taylor, W.J., Jones, P.B., Cornish, J., McQueen, F.M., 2010. Arthritis Res. Ther. 12, R164.
PMID: 20796300; PMCID: PMC2945067
-
Alendronate reduces osteoclast precursors in osteoporosis.
D’Amelio, P., Grimaldi, A., Cristofaro, M.A., Ravazzoli, M., Molinatti, P.A., Pescarmona, G.P., Isaia, G.C., 2010. Osteoporos Int 21, 1741–1750.
PMID: 19949772
-
Id Boufker, H., Lagneaux, L., Najar, M., Piccart, M., Ghanem, G., Body, J.-J., Journé, F., 2010. BMC Cancer 10, 298.
PMID: 20565769; PMCID: PMC3087319
-
Jorde, R., Sneve, M., Torjesen, P.A., Figenschau, Y., Hansen, J.-B., Grimnes, G., 2010. Nutr J 9, 1.
PMID: 20056003; PMCID: PMC2818614
-
Jørgensen, L., Vik, A., Emaus, N., Brox, J., Hansen, J.-B., Mathiesen, E., Vestergaard, P., 2010. Osteoporos Int 21, 931–938.
PMID: 19701599
-
Serum bone turnover markers may be involved in the metastatic potential of lung cancer patients.
Karapanagiotou, E.M., Terpos, E., Dilana, K.D., Alamara, C., Gkiozos, I., Polyzos, A., Syrigos, K.N., 2010. Med. Oncol. 27, 332–338.
PMID: 19373566
-
Lieb, W., Gona, P., Larson, M.G., Massaro, J.M., Lipinska, I., Keaney, J.F., Rong, J., Corey, D., Hoffmann, U., Fox, C.S., Vasan, R.S., Benjamin, E.J., O’Donnell, C.J., Kathiresan, S., 2010. Arterioscler. Thromb. Vasc. Biol. 30, 1849–1854.
PMID: 20448212; PMCID: PMC3039214
-
Paternoster, L., Lorentzon, M., Vandenput, L., Karlsson, M.K., Ljunggren, O., Kindmark, A., Mellstrom, D., Kemp, J.P., Jarett, C.E., Holly, J.M.P., Sayers, A., St Pourcain, B., Timpson, N.J., Deloukas, P., Davey Smith, G., Ring, S.M., Evans, D.M., Tobias, J.H., Ohlsson, C., 2010. PLoS Genet. 6, e1001217.
PMID: 21124946; PMCID: PMC2987837
-
Rauchenzauner, M., Griesmacher, A., Tatarczyk, T., Haberlandt, E., Strasak, A., Zimmerhackl, L.-B., Falkensammer, G., Luef, G., Högler, W., 2010. Dev Med Child Neurol 52, 283–288.
PMID: 19709134
-
Roato, I., Porta, F., Mussa, A., D’Amico, L., Fiore, L., Garelli, D., Spada, M., Ferracini, R., 2010. PLoS ONE 5, e14167.
PMID: 21152388; PMCID: PMC2994752
-
Voskaridou, E., Christoulas, D., Papatheodorou, A., Bratengeier, C., Plata, E., Kaliontzi, D., Woloszczuk, W., Terpos, E., 2010, in: Blood. 1010
-
Serum RANKL, osteoprotegerin (OPG), and RANKL/OPG ratio in nephrotic children.
Wasilewska, A., Rybi-Szuminska, A., Zoch-Zwierz, W., 2010. Pediatr. Nephrol. 25, 2067–2075.
PMID: 20602239; PMCID: PMC2923718
-
Faienza, M.F., Brunetti, G., Colucci, S., Piacente, L., Ciccarelli, M., Giordani, L., Del Vecchio, G.C., D’Amore, M., Albanese, L., Cavallo, L., Grano, M., 2009. J. Clin. Endocrinol. Metab. 94, 2269–2276.
PMID: 19401376
-
González-Calvin, J.L., Mundi, J.L., Casado-Caballero, F.J., Abadia, A.C., Martin-Ibañez, J.J., 2009. J. Clin. Endocrinol. Metab. 94, 4844–4850.
PMID: 19897681
-
Guañabens, N., Enjuanes, A., Alvarez, L., Peris, P., Caballería, L., Jesús Martínez de Osaba, M., Cerdá, D., Monegal, A., Pons, F., Parés, A., 2009. J. Bone Miner. Metab. 27, 347–354.
PMID: 19229472
-
Relationships between OPG, RANKL, bone metabolism, and bone mineral density in biliary atresia.
Honsawek, S., Chaiwatanarat, T., Vejchapipat, P., Chongsrisawat, V., Thawornsuk, N., Poovorawan, Y., 2009. Pediatr. Surg. Int. 25, 261–267.
PMID: 19184056
-
A 246-km continuous running race causes significant changes in bone metabolism.
Kerschan-Schindl, K., Thalmann, M., Sodeck, G.H., Skenderi, K., Matalas, A.L., Grampp, S., Ebner, C., Pietschmann, P., 2009. Bone 45, 1079–1083.
PMID: 19665602
-
Kim, J.G., Kim, H., Jee, B.C., Suh, C.S., Choi, Y.M., Moon, S.Y., 2009. J. Korean Med. Sci. 24, 443–447.
PMID: 19543507; PMCID: PMC2698190
-
Increased serum OPG in atrophic nonunion shaft fractures.
Marchelli, D., Piodi, L.P., Corradini, C., Parravicini, L., Verdoia, C., Ulivieri, F.M., 2009. J Orthop Traumatol 10, 55–58.
PMID: 19484355; PMCID: PMC2688591
-
Mencej-Bedrac, S., Prezelj, J., Kocjan, T., Teskac, K., Ostanek, B., Smelcer, M., Marc, J., 2009. J. Mol. Endocrinol. 42, 239–247.
PMID: 19131500
-
Prostate cancer promotes CD11b positive cells to differentiate into osteoclasts.
Mizutani, K., Sud, S., Pienta, K.J., 2009. J. Cell. Biochem. 106, 563–569.
PMID: 19170075; PMCID: PMC2776620
-
Nenseter, M.S., Ueland, T., Retterstøl, K., Strøm, E., Mørkrid, L., Landaas, S., Ose, L., Aukrust, P., Holven, K.B., 2009. Stroke 40, 241–247.
PMID: 19008470
-
Polyzos, S.A., Anastasilakis, A.D., Efstathiadou, Z., Kita, M., Litsas, I., Avramidis, A., Arsos, G., Moralidis, E., Gerou, S., Pavlidou, V., Papatheodorou, A., Terpos, E., 2009. Hormone and Metabolic Research 41, 846–850.
-
Rauner, M., Stupphann, D., Haas, M., Fert, I., Glatigny, S., Sipos, W., Breban, M., Pietschmann, P., 2009. J. Rheumatol. 36, 120–126.
PMID: 19040304
-
Rendina, D., De Filippo, G., Tauchmanovà, L., Insabato, L., Muscariello, R., Gianfrancesco, F., Esposito, T., Cioffi, M., Colao, A., Strazzullo, P., Mossetti, G., 2009. Calcif. Tissue Int. 85, 293–300.
PMID: 19763378
-
Sauer, A.V., Mrak, E., Hernandez, R.J., Zacchi, E., Cavani, F., Casiraghi, M., Grunebaum, E., Roifman, C.M., Cervi, M.C., Ambrosi, A., Carlucci, F., Roncarolo, M.G., Villa, A., Rubinacci, A., Aiuti, A., 2009. Blood 114, 3216–3226.
PMID: 19633200
-
Syversen, S.W., Goll, G.L., van der Heijde, D., Landewé, R., Gaarder, P.I., Odegård, S., Haavardsholm, E.A., Kvien, T.K., 2009. J. Rheumatol. 36, 266–272.
PMID: 19132792
-
Syversen, U., Stunes, A.K., Gustafsson, B.I., Obrant, K.J., Nordsletten, L., Berge, R., Thommesen, L., Reseland, J.E., 2009. BMC Endocr Disord 9, 10.
PMID: 19331671; PMCID: PMC2678137
-
Terpos, E., Christoulas, D., Katodritou, E., Bratengeier, C., Lindner, B., Harmelin, S., Hawa, G., Boutsikas, G., Migkou, M., Gavriatopoulou, M., 2009
-
Terpos, E., Kiagia, M., Karapanagiotou, E.M., Charpidou, A., Dilana, K.D., Nasothimiou, E., Harrington, K.J., Polyzos, A., Syrigos, K.N., 2009. Anticancer Res. 29, 1651–1657
PMID: 19443381
-
Turk, N., Cukovic-Cavka, S., Korsic, M., Turk, Z., Vucelic, B., 2009. Eur J Gastroenterol Hepatol 21, 159–166.
PMID: 19098682
-
Anastasilakis, A.D., Goulis, D.G., Polyzos, S.A., Gerou, S., Pavlidou, V., Koukoulis, G., Avramidis, A., 2008. Eur. J. Endocrinol. 158, 411–415.
PMID: 18299476
-
Bakhireva, L.N., Laughlin, G.A., Bettencourt, R., Barrett-Connor, E., 2008. J. Clin. Endocrinol. Metab. 93, 2009–2012.
PMID: 18319315; PMCID: PMC2386279
-
D’Amelio, P., Grimaldi, A., Di Bella, S., Tamone, C., Brianza, S.Z.M., Ravazzoli, M.G.A., Bernabei, P., Cristofaro, M.A., Pescarmona, G.P., Isaia, G., 2008. J. Bone Miner. Res. 23, 373–379.
PMID: 17967134
-
Serum osteoprotegerin concentrations are decreased in women with the polycystic ovary syndrome.
Escobar-Morreale, H.F., Botella-Carretero, J.I., Martínez-García, M.A., Luque-Ramírez, M., Alvarez-Blasco, F., San Millán, J.L., 2008. Eur. J. Endocrinol. 159, 225–232.
PMID: 18579554
-
Bone markers predict cardiovascular events in chronic kidney disease.
Fahrleitner-Pammer, A., Herberth, J., Browning, S.R., Obermayer-Pietsch, B., Wirnsberger, G., Holzer, H., Dobnig, H., Malluche, H.H., 2008. J. Bone Miner. Res. 23, 1850–1858.
PMID: 18597636
-
Constitutional thinness: unusual human phenotype of low bone quality.
Galusca, B., Zouch, M., Germain, N., Bossu, C., Frere, D., Lang, F., Lafage-Proust, M.-H., Thomas, T., Vico, L., Estour, B., 2008. J. Clin. Endocrinol. Metab. 93, 110–117.
PMID: 17956951
-
Gendron, S., Boisvert, M., Chetoui, N., Aoudjit, F., 2008. Immunology 125, 359–369.
PMID: 18479350; PMCID: PMC2669139
-
Modifying RANKL/OPG mRNA expression in differentiating and growing human primary osteoblasts.
Giner, M., Montoya, M.J., Vázquez, M.A., Rios, M.J., Moruno, R., Miranda, M.J., Pérez-Cano, R., 2008. Horm. Metab. Res. 40, 869–874.
PMID: 18932123
-
Serum levels of receptor activator of nuclear factor kappaB ligand (RANKL) in healthy women and men.
Kerschan-Schindl, K., Wendlova, J., Kudlacek, S., Gleiss, A., Woloszczuk, W., Pietschmann, P., 2008. Exp. Clin. Endocrinol. Diabetes 116, 491–495.
PMID: 18072013
-
Kwan Tat, S., Pelletier, J.-P., Lajeunesse, D., Fahmi, H., Lavigne, M., Martel-Pelletier, J., 2008. Clin. Exp. Rheumatol. 26, 295–304
PMID: 18565252; PMCID: PMC5247261
-
Increased augmentation index and central aortic blood pressure in osteoporotic postmenopausal women.
Mangiafico, R.A., Alagona, C., Pennisi, P., Parisi, N., Mangiafico, M., Purrello, F., Fiore, C.E., 2008. Osteoporos Int 19, 49–56.
PMID: 17676381
-
Morishita, M., Nagashima, M., Wauke, K., Takahashi, H., Takenouchi, K., 2008. J. Rheumatol. 35, 407–413
PMID: 18260178
-
Nakajima, R., Yamaguchi, M., Kojima, T., Takano, M., Kasai, K., 2008. J. Periodont. Res. 43, 168–173.
PMID: 18302618
-
Nakchbandi, I.A., Lang, R., Kinder, B., Insogna, K.L., 2008. J. Clin. Endocrinol. Metab. 93, 967–973.
PMID: 18073309; PMCID: PMC2266956
-
Pilichou, A., Papassotiriou, I., Michalakakou, K., Fessatou, S., Fandridis, E., Papachristou, G., Terpos, E., 2008. Clin. Biochem. 41, 746–749.
PMID: 18355453
-
Santiago, R.A., Silva, C. a. A., Caparbo, V.F., Sallum, A.M.E., Pereira, R.M.R., 2008. Scand. J. Rheumatol. 37, 40–47.
PMID: 18189194
-
Shroff, R.C., Shah, V., Hiorns, M.P., Schoppet, M., Hofbauer, L.C., Hawa, G., Schurgers, L.J., Singhal, A., Merryweather, I., Brogan, P., Shanahan, C., Deanfield, J., Rees, L., 2008. Nephrol. Dial. Transplant. 23, 3263–3271.
PMID: 18463323
-
Spelling, P., Bonfá, E., Caparbo, V.F., Pereira, R.M.R., 2008. Scand. J. Rheumatol. 37, 439–444.
PMID: 18802807
-
Zhao, H.-Y., Liu, J.-M., Ning, G., Zhao, Y.-J., Chen, Y., Sun, L.-H., Zhang, L.-Z., Xu, M.-Y., Chen, J.-L., 2008. Osteoporos Int 19, 221–226.
PMID: 17703270
-
Andersson, M.K., Lundberg, P., Ohlin, A., Perry, M.J., Lie, A., Stark, A., Lerner, U.H., 2007. Arthritis Res. Ther. 9, R18.
PMID: 17316439; PMCID: PMC1860076
-
Angelopoulos, N.G., Goula, A., Katounda, E., Rombopoulos, G., Kaltzidou, V., Kaltsas, D., Malaktari, S., Athanasiou, V., Tolis, G., 2007. J. Bone Miner. Metab. 25, 60–67.
PMID: 17187195
-
Goranova-Marinova, V., Goranov, S., Pavlov, P., Tzvetkova, T., 2007. Haematologica 92, 1000–1001
PMID: 17606458
-
Helske, S., Kovanen, P.T., Lindstedt, K.A., Salmela, K., Lommi, J., Turto, H., Werkkala, K., Kupari, M., 2007. Eur. J. Heart Fail. 9, 357–363.
PMID: 17254844
-
Soluble receptor activator of nuclear factor-kappa B ligand and risk for cardiovascular disease.
Kiechl, S., Schett, G., Schwaiger, J., Seppi, K., Eder, P., Egger, G., Santer, P., Mayr, A., Xu, Q., Willeit, J., 2007. Circulation 116, 385–391.
PMID: 17620507
-
Kubota, T., Hoshino, M., Aoki, K., Ohya, K., Komano, Y., Nanki, T., Miyasaka, N., Umezawa, K., 2007. Arthritis Res. Ther. 9, R97.
PMID: 17892600; PMCID: PMC2212584
-
Lequerré, T., Jouen, F., Brazier, M., Clayssens, S., Klemmer, N., Ménard, J.-F., Mejjad, O., Daragon, A., Tron, F., Le Loët, X., Vittecoq, O., 2007. Rheumatology 46, 446–453.
PMID: 16899502
-
Monocyte chemotactic protein-1 mediates prostate cancer-induced bone resorption.
Lu, Y., Cai, Z., Xiao, G., Keller, E.T., Mizokami, A., Yao, Z., Roodman, G.D., Zhang, J., 2007. Cancer Res. 67, 3646–3653.
PMID: 17440076
-
Mangiafico, R.A., Malaponte, G., Pennisi, P., Li Volti, G., Trovato, G., Mangiafico, M., Bevelacqua, Y., Mazza, F., Fiore, C.E., 2007. J. Intern. Med. 261, 587–596.
PMID: 17547714
-
Martini, G., Gennari, L., Merlotti, D., Salvadori, S., Franci, M.B., Campagna, S., Avanzati, A., De Paola, V., Valleggi, F., Nuti, R., 2007. Bone 40, 457–463.
PMID: 16979395
-
Changes of OPG and RANKL concentrations in Crohn’s disease after infliximab therapy.
Miheller, P., Muzes, G., Rácz, K., Blázovits, A., Lakatos, P., Herszényi, L., Tulassay, Z., 2007. Inflamm. Bowel Dis. 13, 1379–1384.
PMID: 17663430
-
Expression of cathepsin-K in gingival crevicular fluid of patients with periodontitis.
Mogi, M., Otogoto, J., 2007. Arch. Oral Biol. 52, 894–898.
PMID: 17321485
-
Monegal, A., Navasa, M., Peris, P., Alvarez, L., Pons, F., Rodés, J., Guañabens, N., 2007. Liver Int. 27, 492–497.
PMID: 17403189
-
Mora, S., Zamproni, I., Cafarelli, L., Giacomet, V., Erba, P., Zuccotti, G., Viganò, A., 2007. AIDS 21, 1129–1135.
PMID: 17502723
-
The “lively” cytokines network in beta-Thalassemia Major-related osteoporosis.
Morabito, N., Russo, G.T., Gaudio, A., Lasco, A., Catalano, A., Morini, E., Franchina, F., Maisano, D., La Rosa, M., Plota, M., Crifò, A., Meo, A., Frisina, N., 2007. Bone 40, 1588–1594.
PMID: 17412659
-
Bone structure and metabolism in a rodent model of male senile osteoporosis.
Pietschmann, P., Skalicky, M., Kneissel, M., Rauner, M., Hofbauer, G., Stupphann, D., Viidik, A., 2007. Exp. Gerontol. 42, 1099–1108.
PMID: 17949933
-
Rouster-Stevens, K.A., Langman, C.B., Price, H.E., Seshadri, R., Shore, R.M., Abbott, K., Pachman, L.M., 2007. Arthritis Rheum. 56, 977–983.
PMID: 17328075
-
Stern, A., Laughlin, G.A., Bergstrom, J., Barrett-Connor, E., 2007. Eur. J. Endocrinol. 156, 555–562.
PMID: 17468191; PMCID: PMC2642656
-
Suh, K.T., Lee, S.-S., Hwang, S.H., Kim, S.-J., Lee, J.S., 2007. Eur Spine J 16, 1563–1569.
PMID: 17520299; PMCID: PMC2078303
-
Whyte, M.P., Singhellakis, P.N., Petersen, M.B., Davies, M., Totty, W.G., Mumm, S., 2007. J. Bone Miner. Res. 22, 938–946.
PMID: 17352649
-
Association between phosphate removal and markers of bone turnover in haemodialysis patients.
Albalate, M., de la Piedra, C., Fernández, C., Lefort, M., Santana, H., Hernando, P., Hernández, J., Caramelo, C., 2006. Nephrol. Dial. Transplant. 21, 1626–1632.
PMID: 16490746
-
Ishii, R., Morimoto, A., Ikushima, S., Sugimoto, T., Asami, K., Bessho, F., Kudo, K., Tsunematu, Y., Fujimoto, J., Imashuku, S., 2006. Pediatr Blood Cancer 47, 194–199.
PMID: 16358318
-
IL-7 up-regulates TNF-alpha-dependent osteoclastogenesis in patients affected by solid tumor.
Roato, I., Brunetti, G., Gorassini, E., Grano, M., Colucci, S., Bonello, L., Buffoni, L., Manfredi, R., Ruffini, E., Ottaviani, D., Ciuffreda, L., Mussa, A., Ferracini, R., 2006. PLoS ONE 1, e124.
PMID: 17205128; PMCID: PMC1762428
-
Secchiero, P., Corallini, F., Pandolfi, A., Consoli, A., Candido, R., Fabris, B., Celeghini, C., Capitani, S., Zauli, G., 2006. Am. J. Pathol. 169, 2236–2244.
PMID: 17148684; PMCID: PMC1762477
-
Are activated T cells regulators of bone metabolism in children with Crohn disease?
Sylvester, F.A., Davis, P.M., Wyzga, N., Hyams, J.S., Lerer, T., 2006. J. Pediatr. 148, 461–466.
PMID: 16647405
-
Terpos, E., Heath, D.J., Rahemtulla, A., Zervas, K., Chantry, A., Anagnostopoulos, A., Pouli, A., Katodritou, E., Verrou, E., Vervessou, E.-C., Dimopoulos, M.-A., Croucher, P.I., 2006. Br. J. Haematol. 135, 688–692.
PMID: 17107351
-
Voorzanger-Rousselot, N., Juillet, F., Mareau, E., Zimmermann, J., Kalebic, T., Garnero, P., 2006. Br. J. Cancer 95, 506–514.
PMID: 16880790; PMCID: PMC2360666
-
Abrahamsen, B., Hjelmborg, J.V., Kostenuik, P., Stilgren, L.S., Kyvik, K., Adamu, S., Brixen, K., Langdahl, B.L., 2005. Bone 36, 727–735.
PMID: 15781001
-
Increased bone resorption in HD patients: is it caused by elevated RANKL synthesis?
Avbersek-Luznik, I., Balon, B.P., Rus, I., Marc, J., 2005. Nephrol. Dial. Transplant. 20, 566–570.
PMID: 15665031
-
Bezerra, M.C., Calomeni, G.D., Caparbo, V.F., Gebrim, E.S., Rocha, M.S., Pereira, R.M.R., 2005. Rheumatology 44, 1503–1506.
PMID: 16219645
-
Chan, M.H.M., Wong, K., Chan, I.H.S., Luo, Y.F., Tam, S., Lam, C.W.K., 2005. Pathology 37, 51–55
PMID: 15875734
-
Osteoprotegerin and RANKL in alcoholic liver cirrhosis.
Fábrega, E., Orive, A., García-Suarez, C., García-Unzueta, M., Antonio Amado, J., Pons-Romero, F., 2005. Liver Int. 25, 305–310.
PMID: 15780054
-
Liu, J.M., Zhao, H.Y., Ning, G., Zhao, Y.J., Chen, Y., Zhang, Z., Sun, L.H., Xu, M.-Y., Chen, J.L., 2005. Calcif. Tissue Int. 76, 1–6.
PMID: 15455183
-
Maïmoun, L., Couret, I., Mariano-Goulart, D., Dupuy, A.M., Micallef, J.-P., Peruchon, E., Ohanna, F., Cristol, J.-P., Rossi, M., Leroux, J.-L., 2005. Calcif. Tissue Int. 76, 404–411.
PMID: 15812577
-
Moschen, A.R., Kaser, A., Enrich, B., Ludwiczek, O., Gabriel, M., Obrist, P., Wolf, A.M., Tilg, H., 2005. Gut 54, 479–487.
PMID: 15753532; PMCID: PMC1774465
-
Osteoblast response to thermally oxidized Ti6Al4V alloy.
Saldaña, L., Vilaboa, N., Vallés, G., González-Cabrero, J., Munuera, L., 2005. J Biomed Mater Res A 73, 97–107.
PMID: 15704115
-
Osteoprotegerin and bone turnover markers in heavily pretreated HIV-infected patients.
Seminari, E., Castagna, A., Soldarini, A., Galli, L., Fusetti, G., Dorigatti, F., Hasson, H., Danise, A., Guffanti, M., Lazzarin, A., Rubinacci, A., 2005. HIV Med. 6, 145–150.
PMID: 15876279
-
Terpos, E., Mihou, D., Szydlo, R., Tsimirika, K., Karkantaris, C., Politou, M., Voskaridou, E., Rahemtulla, A., Dimopoulos, M.A., Zervas, K., 2005. Leukemia 19, 1969–1976.
PMID: 16079895
-
Dysregulated osteoprotegerin/RANK ligand/RANK axis in clinical and experimental heart failure.
Ueland, T., Yndestad, A., Øie, E., Florholmen, G., Halvorsen, B., Frøland, S.S., Simonsen, S., Christensen, G., Gullestad, L., Aukrust, P., 2005. Circulation 111, 2461–2468.
PMID: 15883214
-
Zhao, H., Liu, J., Ning, G., Zhao, Y., Zhang, L., Sun, L., Xu, M., Uitterlinden, A.G., Chen, J., 2005. Osteoporos Int 16, 1519–1524.
PMID: 15782282
-
Zojer, N., Brenner, K., Beke, D., Kudlacek, S., Hawa, G., Woloszczuk, W., Hofbauer, L.C., Pecherstorfer, M., 2005. Anticancer Res. 25, 3607–3612
PMID: 16101188
-
Buzi, F., Maccarinelli, G., Guaragni, B., Ruggeri, F., Radetti, G., Meini, A., Mazzolari, E., Cocchi, D., 2004. Clin. Endocrinol. 60, 87–91
PMID: 14678293
-
Colucci, S., Brunetti, G., Rizzi, R., Zonno, A., Mori, G., Colaianni, G., Del Prete, D., Faccio, R., Liso, A., Capalbo, S., Liso, V., Zallone, A., Grano, M., 2004. Blood 104, 3722–3730.
PMID: 15308561
-
Dai, S., Nishioka, K., Yudoh, K., 2004. Ann Rheum Dis 63, 1379–1386.
PMID: 15479886; PMCID: PMC1754791
-
Franchimont, N., Reenaers, C., Lambert, C., Belaiche, J., Bours, V., Malaise, M., Delvenne, P., Louis, E., 2004. Clin. Exp. Immunol. 138, 491–498.
PMID: 15544627; PMCID: PMC1809233
-
Hofbauer, L.C., Schoppet, M., Schüller, P., Viereck, V., Christ, M., 2004. Clin. Endocrinol. 60, 214–219
PMID: 14725683
-
Low bone density and abnormal bone turnover in patients with atherosclerosis of peripheral vessels.
Pennisi, P., Signorelli, S.S., Riccobene, S., Celotta, G., Di Pino, L., La Malfa, T., Fiore, C.E., 2004. Osteoporos Int 15, 389–395.
PMID: 14661073
-
Soluble RANKL and risk of nontraumatic fracture.
Schett, G., Kiechl, S., Redlich, K., Oberhollenzer, F., Weger, S., Egger, G., Mayr, A., Jocher, J., Xu, Q., Pietschmann, P., Teitelbaum, S., Smolen, J., Willeit, J., 2004. JAMA 291, 1108–1113.
PMID: 14996780
-
Skoumal, M., Kolarz, G., Woloszczuk, W., Hawa, G., Klingler, A., 2004. Ann Rheum Dis 63, 216–217.
PMID: 14722219; PMCID: PMC1754876
-
Terpos, E., Politou, M., Szydlo, R., Nadal, E., Avery, S., Olavarria, E., Kanfer, E., Goldman, J.M., Apperley, J.F., Rahemtulla, A., 2004. Leukemia 18, 1420–1426.
PMID: 15215875
-
Alvarez, L., Peris, P., Guañabens, N., Vidal, S., Ros, I., Pons, F., Filella, X., Monegal, A., Muñoz-Gomez, J., Ballesta, A.M., 2003. Arthritis Rheum. 48, 824–828.
PMID: 12632438
-
Chan, B.Y.Y., Buckley, K.A., Durham, B.H., Gallagher, J.A., Fraser, W.D., 2003. Clin. Chem. 49, 2083–2085.
PMID: 14633883
-
Familial severe congenital neutropenia associated with infantile osteoporosis: a new entity.
Elhasid, R., Hofbauer, L.C., Ish-Shalom, S., Ben-Arush, M., Koc, O., Rowe, J.M., Etzioni, A., 2003. Am. J. Hematol. 72, 34–37.
PMID: 12508266
-
Immunoassay for soluble RANKL (receptor activator of NF-kappaB ligand) in serum.
Hawa, G., Brinskelle-Schmal, N., Glatz, K., Maitzen, S., Woloszczuk, W., 2003. Clin. Lab. 49, 461–463
PMID: 14572201
-
Osteoprotegerin and sRANKL serum levels in multiple myeloma patients.
Kruk, B., Kraj, M., Centkowski, P., Sokołowska, U., 2003. Cent Eur J Immunol 27, 129–135
-
Krystufkova, O., Niederlova, J., Senolt, V., Hladikova, M., Ruzickova, S., Vencovsky, J., 2003. Arthritis Res Ther 5, 102.
PMID: null; PMCID: PMC2833669
-
Schoppet, M., Schaefer, J.R., Hofbauer, L.C., 2003. Circulation 107, e76;
PMID: 12654623
-
High serum osteoprotegerin and low RANKL in primary biliary cirrhosis.
Szalay, F., Hegedus, D., Lakatos, P.L., Tornai, I., Bajnok, E., Dunkel, K., Lakatos, P., 2003. J. Hepatol. 38, 395–400
PMID: 12663228
-
Terpos, E., Samarkos, M., Meletis, C., Apostolidou, E., Tsironi, M., Korovesis, K., Mavrogianni, D., Viniou, N., Meletis, J., 2003a. Int. J. Hematol. 78, 344–348
PMID: 14686493
-
Terpos, E., Szydlo, R., Apperley, J.F., Hatjiharissi, E., Politou, M., Meletis, J., Viniou, N., Yataganas, X., Goldman, J.M., Rahemtulla, A., 2003. Blood 102, 1064–1069.
PMID: 12689925
-
von Tirpitz, C., Epp, S., Klaus, J., Mason, R., Hawa, G., Brinskelle-Schmal, N., Hofbauer, L.C., Adler, G., Kratzer, W., Reinshagen, M., 2003. Eur J Gastroenterol Hepatol 15, 1165–1170.
PMID: 14560148
-
Pamidronate is an effective treatment for osteoporosis in patients with beta-thalassaemia.
Voskaridou, E., Terpos, E., Spina, G., Palermos, J., Rahemtulla, A., Loutradi, A., Loukopoulos, D., 2003. Br. J. Haematol. 123, 730–737
PMID: 14616979
-
Giuliani, N., Colla, S., Sala, R., Moroni, M., Lazzaretti, M., La Monica, S., Bonomini, S., Hojden, M., Sammarelli, G., Barillè, S., Bataille, R., Rizzoli, V., 2002. Blood 100, 4615–4621.
PMID: 12393684
-
Decreased bone density, elevated serum osteoprotegerin, and beta-cross-laps in Wilson disease.
Hegedus, D., Ferencz, V., Lakatos, P.L., Meszaros, S., Lakatos, P., Horvath, C., Szalay, F., 2002. J. Bone Miner. Res. 17, 1961–1967.
PMID: 12412803
-
Osteoprotegerin deficiency and juvenile Paget’s disease.
Whyte, M.P., Obrecht, S.E., Finnegan, P.M., Jones, J.L., Podgornik, M.N., McAlister, W.H., Mumm, S., 2002. N. Engl. J. Med. 347, 175–184.
PMID: 12124406
-
Elevated levels of osteoprotegerin (OPG) and hepatocyte growth factor (HGF) in rheumatoid arthritis.
Feuerherm, A.J., Borset, M., Seidel, C., Sundan, A., Leistad, L., Ostensen, M., Faxvaag, A., 2001. Scand. J. Rheumatol. 30, 229–234
PMID: 11578019
 Download biomedica product list 2018
Download biomedica product list 2018